Page 129 - IJB-9-4
P. 129
International Journal of Bioprinting 3D bioprinted models in pediatric tumors
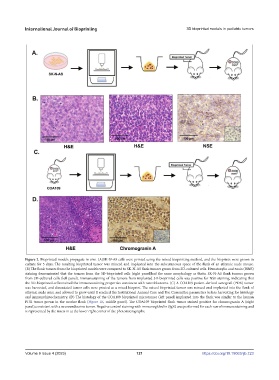
Figure 2. Bioprinted models propagate in vivo. (A)SK-N-AS cells were printed using the mixed bioprinting method, and the bioprints were grown in
culture for 5 days. The resulting bioprinted tumor was minced and implanted into the subcutaneous space of the flank of an athymic nude mouse.
(B) The flank tumors from the bioprinted models were compared to SK-N-AS flank tumors grown from 2D-cultured cells. Hematoxylin and eosin (H&E)
staining demonstrated that the tumors from the 3D-bioprinted cells (right panel)had the same morphology as thatin SK-N-AS flank tumors grown
from 2D-cultured cells (left panel). Immunostaining of the tumors from implanted 3D-bioprinted cells was positive for NSE staining, indicating that
the 3D-bioprinted cellsretained the immunostaining properties consistent with neuroblastoma. (C) A COA109 patient-derived xenograft (PDX) tumor
was harvested, and dissociated tumor cells were printed as a mixed bioprint. The mixed bioprinted tumor was minced and implanted into the flank of
athymic nude mice and allowed to grow until it reached the Institutional Animal Care and Use Committee parameters before harvesting for histology
and immunohistochemistry. (D) The histology of the COA109 bioprinted microtumor (left panel) implanted into the flank was similar to the human
PDX tumor grown in the murine flank (Figure 1E, middle panel). The COA109 bioprinted flank tumor stained positive for chromogranin A (right
panel),consistent with a neuroendocrine tumor. Negative control staining with immunoglobulin (Ig)G was performed for each run of immunostaining and
is represented by the insets in at the lower right corner of the photomicrographs.
Volume 9 Issue 4 (2023) 121 https://doi.org/10.18063/ijb.723