Page 130 - IJB-9-4
P. 130
International Journal of Bioprinting 3D bioprinted models in pediatric tumors
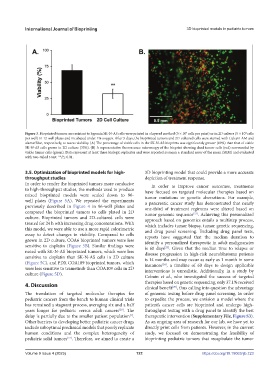
Figure 3. Bioprinted tumors are resistant to hypoxia.SK-N-AS cells were printed in a layered method (5 × 10 cells per print) or in 2D culture (5 × 10 cells
5
4
per well) in 12-well plates and incubated under 1% oxygen. After 5 days, the bioprinted tumors and 2D-cultured cells were stained with Calcein AM and
alamarBlue, respectively, to assess viability. (A) The percentage of viable cells in the SK-N-AS bioprints was significantly greater (69%) than that of viable
SK-N-AS cells grown in 2D culture (33%). (B) A representative fluorescence microscopy of the bioprint showing dead tumor cells (red) surrounded by
viable tumor cells (green). Data represent at least three biologic replicates and were reported as mean ± standard error of the mean (SEM) and evaluated
with two-tailed t-test. **P≤ 0.01.
3.5. Optimization of bioprinted models for high- 3D bioprinting model that could provide a more accurate
throughput studies depiction of treatment response.
In order to render the bioprinted tumors more conducive In order to improve cancer outcomes, treatments
to high-throughput studies, the methods used to produce have focused on targeted molecular therapies based on
mixed bioprinted models were scaled down to 96- tumor mutations or genetic aberrations. For example,
well plates (Figure 5A). We repeated the experiments a pancreatic cancer study has demonstrated that nearly
previously described in Figure 4 in 96-well plates and one-third of treatment regimens were altered based on
compared the bioprinted tumors to cells plated in 2D tumor genomic sequence . Achieving this personalized
[22]
culture. Bioprinted tumors and 2D-cultured cells were approach based on genomics entails a multistep process,
treated for 24 h with increasing drug concentrations. With which includes tumor biopsy, tumor genetic sequencing,
this model, we were able to use a more rapid colorimetric and drug panel screening. Excluding drug panel tests,
assay to detect changes in viability. Compared to cells reports have suggested that the median duration to
grown in 2D culture, COA6 bioprinted tumors were less identify a personalized therapeutic in adult malignancies
sensitive to cisplatin (Figure 5B). Similar findings were is 60 days . Given that the median time to relapse or
[23]
noted with SK-N-AS bioprinted tumors, which were less disease progression in high-risk neuroblastoma patients
sensitive to cisplatin than SK-N-AS cells in 2D culture is 14 months and may occur as early as 1 month in some
(Figure 5C), and PDX COA109 bioprinted tumors, which instances , a timeline of 60 days to design applicable
[24]
were less sensitive to trametinib than COA109 cells in 2D interventions is unrealistic. Additionally, in a study by
culture (Figure 5D). Cobain et al., who investigated the success of targeted
4. Discussion therapies based on genetic sequencing, only 37.1% received
clinical benefit , thus calling into question the advantage
[25]
The translation of targeted molecular therapies for of genomic testing before drug panel screening. In order
pediatric cancers from the bench to human clinical trials to expedite the process, we envision a model where the
has remained a stagnant process, averaging six and a half patient’s cancer cells are bioprinted and undergo high-
years longer for pediatric versus adult cancers . The throughput testing with a drug panel to identify the best
[20]
delay is partially due to the smaller patient population . therapeutic intervention (Supplementary File, Figure S3).
[1]
Other barriers to developing better pediatric cancer drugs As an ongoing area of research for our lab, we have yet to
include suboptimal preclinical models that poorly replicate directly print cells from patients. However, in the current
human conditions and the complex heterogeneity of study, we focused on demonstrating the feasibility of
pediatric solid tumors . Therefore, we aimed to create a bioprinting pediatric tumors that recapitulate the tumor
[21]
Volume 9 Issue 4 (2023) 122 https://doi.org/10.18063/ijb.723