Page 132 - IJB-9-4
P. 132
International Journal of Bioprinting 3D bioprinted models in pediatric tumors
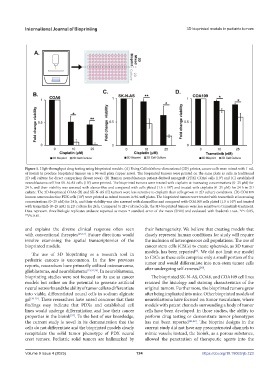
Figure 5. High-throughput drug testing using bioprinted models. (A) Using Cellink’sthree-dimensional (3D) printer, cancer cells were mixed with 1 mL
of bioink to produce bioprinted tumors on a 96-well plate (upper rows). The bioprinted tumors were printed on the same plate as cells in traditional
2D cell culture for direct comparison (lower rows). (B) Human neuroblastoma patient-derived xenograft (PDX) COA6 cells (10 ) and (C) established
7
neuroblastoma cell line SK-N-AS cells (10 ) were printed. The bioprinted tumors were treated with cisplatin at increasing concentrations (0–25 µM) for
7
24 h, and their viability was assessed with alamarBlue and compared with cells plated (1.5 × 10 ) and treated with cisplatin (0–25 µM) for 24 h in 2D
4
culture. The 3D-bioprinted COA6 (B) and SK-N-AS (C) tumors were less sensitive to cisplatin than cells grown in 2D culture conditions. (D) COA109
human neuroendocrine PDX cells (10 ) were printed as mixed tumors in 96-well plates. The bioprinted tumors were treated with trametinib at increasing
7
concentrations (0–25 nM) for 24 h, and their viability was also assessed with alamarBlue and compared with COA109 cells plated (1.5 × 10 ) and treated
4
with trametinib (0–25 nM) in 2D culture for 24 h. Compared to 2D-cultured cells, the 3D-bioprinted tumors were less sensitive to trametinib treatment.
Data represent three biologic replicates andwere reported as mean ± standard error of the mean (SEM) and evaluated with Student’s t-test. *P≤ 0.05,
**P≤ 0.01.
and explains the diverse clinical response often seen their heterogeneity. We believe that creating models that
with conventional therapies [25,33] . Future directions would closely represent human conditions for study will require
involve examining the spatial transcriptomics of the the inclusion of heterogeneous cell populations. The use of
bioprinted models. cancer stem cells (CSCs) to create spheroids, as 3D tumor
models, has been reported . We did not limit our model
[6]
The use of 3D bioprinting as a research tool in
pediatric cancers is uncommon. In the few previous to CSCs as these cells comprise only a small portion of the
tumor and would differentiate into non-stem tumor cells
reports, researchers have primarily utilized osteosarcoma, after undergoing self-renewal .
[40]
glioblastoma, and neuroblastoma [13,34,35] . In neuroblastoma,
bioprinting studies were not focused on its use as cancer The bioprinted SK-N-AS, COA6, and COA109 cell lines
models but rather on the potential to generate artificial retained the histology and staining characteristics of the
neural networks and the ability of tumor cells to differentiate original tumors. Furthermore, the bioprinted tumors grew
into viable, differentiated neural cells in sodium alginate after being implanted into mice. Other bioprinted models of
gel [36-39] . These researchers have raised concerns that their neuroblastoma have focused on tumor vasculature, where
findings may indicate that PDXs and established cell models with patent channels surrounding a body of tumor
lines would undergo differentiation and lose their cancer cells have been developed. In those studies, the ability to
properties in the bioink . To the best of our knowledge, perform drug testing or demonstrate tumor phenotypes
[37]
the current study is novel in its demonstration that the has not been reported [41,42] . The bioprint designs in the
cells do not differentiate and the bioprinted models clearly current study did not have any preconstructed channels to
recapitulate the solid tumor phenotype of PDX neural mimic vessels; instead, the bioink, as a porous substance,
crest tumors. Pediatric solid tumors are hallmarked by allowed the penetration of therapeutic agents into the
Volume 9 Issue 4 (2023) 124 https://doi.org/10.18063/ijb.723