Page 175 - IJB-9-4
P. 175
International Journal of Bioprinting Multi-scale vascularization strategy for 3D-bioprinted tissue
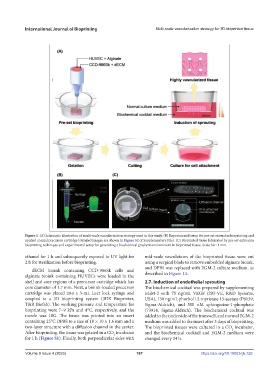
Figure 1. (A) Schematic illustration of multi-scale vascularization strategy used in this study. (B) Experimental setup for pre-set extrusion bioprinting and
applied coaxial precursor cartridge (detailed images are shown in Figure S2 of Supplementary File). (C) Bioprinted tissue fabricated by pre-set extrusion
bioprinting technique and experimental setup for generating a biochemical gradient environment in bioprinted tissue. Scale bar: 1 mm.
ethanol for 1 h and subsequently exposed to UV light for mid-scale vasculatures of the bioprinted tissue were cut
2 h for sterilization before bioprinting. using a surgical blade to remove embedded alginate bioink,
and DPBS was replaced with EGM-2 culture medium, as
dECM bioink containing CCD-986Sk cells and
alginate bioink containing HUVECs were loaded in the described in Figure 1A.
shell and core regions of a precursor cartridge which has 2.7. Induction of endothelial sprouting
core diameter of 1.7 mm. Next, a bioink-loaded precursor The biochemical cocktail was prepared by supplementing
cartridge was placed into a 3-mL Luer lock syringe and EGM-2 with 75 ng/mL VEGF (293-VE, R&D Systems,
coupled to a 3D bioprinting system (3DX Bioprinter, USA), 150 ng/mL phorbol 12-myristate 13-acetate (P8139,
T&R Biofab). The working pressure and temperature for Sigma-Aldrich), and 500 nM sphingosine-1-phosphate
bioprinting were 7–9 kPa and 4°C, respectively, and the (73914, Sigma-Aldrich). The biochemical cocktail was
nozzle was 18G. The tissue was printed into an insert added to the underside of the transwell, and normal EGM-2
containing 25°C DPBS to a size of 10 × 10 × 1.6 mm and a medium was added to the insert after 3 days of bioprinting.
two-layer structure with a diffusion channel in the center. The bioprinted tissues were cultured in a CO incubator,
2
After bioprinting, the tissue was gelated in a CO incubator and the biochemical cocktail and EGM-2 medium were
2
for 1 h (Figure S1). Finally, both perpendicular sides with changed every 24 h.
Volume 9 Issue 4 (2023) 167 https://doi.org/10.18063/ijb.726