Page 177 - IJB-9-4
P. 177
International Journal of Bioprinting Multi-scale vascularization strategy for 3D-bioprinted tissue
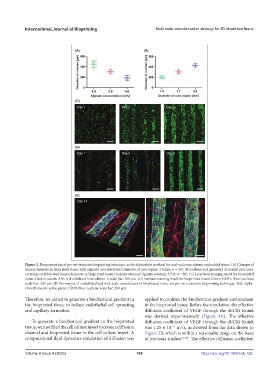
Figure 2. Demonstration of pre-set extrusion bioprinting technique as the fabrication method for mid-scale vasculature-embedded tissue. (A) Changes of
lumen diameter in bioprinted tissue with alginate concentration (diameter of core region: 3.5 mm, n = 30). (B) Influence of geometry of coaxial precursor
cartridge on fabricated lumen diameter in bioprinted tissue (concentration of alginate solution: 3.5%, n = 30). (C) Live/dead staining result for bioprinted
tissue. Green: calcein-AM; red: ethidium homodimer-1; scale bar: 300 μm. (D) Immunostaining result for bioprinted tissue. Green: CD31; Blue: nucleus;
scale bar: 300 μm. (E) Formation of endothelialized mid-scale vasculatures in bioprinted tissue via pre-set extrusion bioprinting technique. Red: alpha-
smooth muscle actin; green: CD31; blue: nucleus; scale bar: 200 μm.
Therefore, we aimed to generate a biochemical gradient in applied to confirm the biochemical gradient environment
the bioprinted tissue to induce endothelial cell sprouting in the bioprinted tissue. Before the simulation, the effective
and capillary formation. diffusion coefficient of VEGF through the dECM bioink
was derived experimentally (Figure 3A). The effective
To generate a biochemical gradient in the bioprinted diffusion coefficient of VEGF through the dECM bioink
tissue, we modified the cell culture insert to create a diffusion was 1.25 × 10 m /s, as derived from the data shown in
2
−11
channel and bioprinted tissue in the cell culture insert. A Figure 3B, which is within a reasonable range on the basis
computational fluid dynamics simulation of diffusion was of previous studies [17,18] . The effective diffusion coefficient
Volume 9 Issue 4 (2023) 169 https://doi.org/10.18063/ijb.726