Page 275 - IJB-9-4
P. 275
International Journal of Bioprinting 3D acoustically assembled cell spheroids with high-throughput
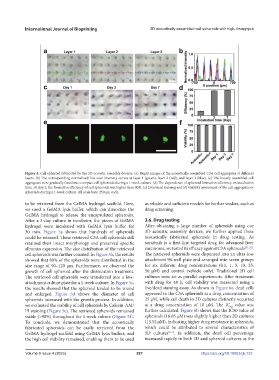
Figure 4. Cell spheroid fabricated by the 3D acoustic assembly devices. (a) Bright images of the acoustically assembled C3A cell aggregates at different
layers. (b) The corresponding normalized line scan intensity curves at layer 1 (green), layer 2 (red), and layer 3 (blue). (c) The loosely assembled cell
aggregates were gradually fused into compact cell spheroids during a 1-week culture. (d) The dependence of spheroid formation efficiency on incubation
time. At day 3, the formation efficiency of cell spheroids was higher than 90%. (e) Live/dead staining and (f) viability assessment of the cell aggregates or
spheroids during a 1-week culture. All scale bars: 250 μm each.
to be retrieved from the GelMA hydrogel scaffold. Here, as reliable and sufficient models for further studies, such as
we used a GelMA lysis buffer, which can dissociate the drug screening.
GelMA hydrogel to release the encapsulated spheroids.
After a 3-day culture in incubator, the pieces of GelMA 3.6. Drug testing
hydrogel were incubated with GelMA lysis buffer for After obtaining a large number of spheroids using our
30 min. Figure 5a shows that hundreds of spheroids 3D acoustic assembly devices, we further applied these
could be released. These retrieved C3A cell spheroids still acoustically fabricated spheroids in drug testing. As
retained their intact morphology and preserved specific sorafenib is a first-line targeted drug for advanced liver
albumin expression. The size distribution of the retrieved carcinoma, we tested its efficacy against C3A spheroids [51,52] .
cell spheroids was further counted. In Figure 5b, the results The retrieved spheroids were dispensed into an ultra-low
showed that 80% of the spheroids were distributed in the attachment 96-well plate and arranged into seven groups
size range of 90–120 μm. Furthermore, we observed the for six different drug concentrations (0.1, 1, 5, 10, 25,
growth of cell spheroid after the dissociation treatment. 50 μM) and control (vehicle only). Traditional 2D cell
The retrieved cell spheroids were transferred into a low- cultures were set as parallel experiments. After treatment
attachment culture plate for a 1-week culture. In Figure 5c, with drug for 48 h, cell viability was measured using a
the results showed that the spheroid tended to be round live/dead staining assay. As shown in Figure 6a, dead cells
and enlarged. Figure 5d shows the diameter of cell appeared in the C3A spheroids at a drug concentration of
spheroids increased with the growth process. In addition, 25 μM, while cell death in 2D cultures distinctly occurred
we evaluated the viability of cell spheroids by Calcein-AM/ at a drug concentration of 10 μM. The IC value was
50
PI staining (Figure 5e). The retrieved spheroids remained further calculated. Figure 6b shows that the IC50 value of
viable (>90%) throughout the 1-week culture (Figure 5f). spheroids (16.03 μM) was slightly higher than 2D cultures
To conclude, we demonstrated that the acoustically (13.52 μM), indicating higher drug resistance in spheroids,
fabricated spheroids can be easily retrieved from the which could be attributed to several characteristics of
GelMA hydrogel scaffold using GelMA lysis buffers, and 3D cultures [4-7] . In addition, the dead cell percentage
the high cell viability remained, enabling them to be used increased rapidly in both 2D and spheroid cultures as the
Volume 9 Issue 4 (2023) 267 https://doi.org/10.18063/ijb.733