Page 30 - IJB-9-5
P. 30
International Journal of Bioprinting 3D bioprinted vascularized tissue models
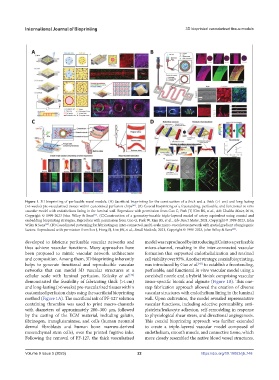
Figure 1. 3D bioprinting of perfusable vessel models. (A) Sacrificial bioprinting for the construction of a thick and a thick (>1 cm) and long-lasting
[42]
(>6 weeks) pre-vascularized tissues within customized perfusion chips . (B) Coaxial bioprinting of a freestanding, perfusable, and functional in vitro
vascular model with endothelium lining in the luminal wall. Reproduce with permission from Gao G, Park JY, Kim BS, et al., Adv Healthc Mater, 2018,
[43]
Copyright © 1999-2023 John Wiley & Sons . (C)Construction of a geometry-tunable triple-layered model of artery equivalent using coaxial and
embedding bioprinting strategies. Reproduce with permission from Gao G, Park W, Kim BS, et al., Adv Funct Mater, 2021, Copyright © 1999-2023, John
[40]
Wiley & Sons . (D) Coordinated patterning for fabricating an inter-connected, multi-scale micro-vasculature network with spatial gradient of angiogenic
[44]
factors. Reproduced with permission from Son J, Hong SJ, Lim JW, et al., Small Methods, 2021, Copyright © 1999-2023, John Wiley & Sons .
developed to fabricate perfusable vascular networks and model was reproduced by introducing ECs into a perfusable
thus achieve vascular functions. Many approaches have micro-channel, resulting in the inter-connected vascular
been proposed to mimic vascular network architecture formation that supported endothelialization and retained
and composition. Among them, 3D bioprinting inherently cell viability over 95%. Another strategy, coaxial bioprinting,
helps to generate functional and reproducible vascular was introduced by Gao et al. to establish a freestanding,
[43]
networks that can model 3D vascular structures at a perfusable, and functional in vitro vascular model using a
cellular scale with luminal perfusion. Kolesky et al. core/shell nozzle and a hybrid bioink comprising vascular
[42]
demonstrated the feasibility of fabricating thick (>1 cm) tissue-specific bioink and alginate (Figure 1B). This one-
and long-lasting (>6 weeks) pre-vascularized tissues within step fabrication approach allowed the creation of diverse
customized perfusion chips using the sacrificial bioprinting vascular structures with endothelium lining in the luminal
method (Figure 1A). The sacrificial ink of PF-127 solution wall. Upon cultivation, the model revealed representative
containing thrombin was used to print macro-channels vascular functions, including selective permeability, anti-
with diameters of approximately 200–300 µm, followed platelets/leukocyte adhesion, self-remodeling in response
by the casting of the ECM material, including gelatin, to physiological shear stress, and directional angiogenesis.
fibrinogen, transglutaminase, and cells (human neonatal This coaxial bioprinting approach was further extended
dermal fibroblasts and human bone marrow-derived to create a triple-layered vascular model composed of
mesenchymal stem cells), over the printed fugitive inks. endothelium, smooth muscle, and connective tissue, which
Following the removal of PF-127, the thick vascularized more closely resembled the native blood vessel structures.
Volume 9 Issue 5 (2023) 22 https://doi.org/10.18063/ijb.748