Page 32 - IJB-9-5
P. 32
International Journal of Bioprinting 3D bioprinted vascularized tissue models
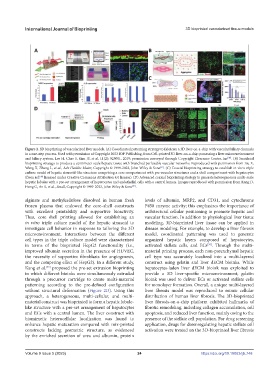
Figure 2. 3D bioprinting of vascularized liver models. (A) Coordinated patterning strategy to fabricate a 3D liver-on-a-chip with vascular/biliary channels
in a one-step process. Used with permission of Copyright 2023 IOP Publishing, from Cell-printed 3D liver-on-a-chip possessing a liver microenvironment
and biliary system, Lee H, Chae S, Kim JY, et al, 11(2): 025001, 2019; permission conveyed through Copyright Clearance Center, Inc . (B) Sacrificial
[46]
bioprinting strategy to produce a centimeter-scale hepatic tissue with branched perfusable vascular networks. Reproduced with permission from Liu X,
Wang X, Zhang L, et al., Adv Healthc Mater, Copyright © 1999-2023, John Wiley & Sons . (C) Coaxial bioprinting strategy to establish in vitro triple
[47]
culture model of hepatic sinusoid-like structure comprising a core compartment with pre-vascular structures and a shell compartment with hepatocytes
(from ref. licensed under Creative Commons Attribution 4.0 license). (D) Advanced coaxial bioprinting strategy to generate heterogeneous multi-scale
[48]
hepatic lobules with a pre-set arrangement of hepatocytes and endothelial cells with a central lumen. Images reproduced with permission from Kang D,
Hong G, An S, et al., Small, Copyright © 1999-2023, John Wiley & Sons .
[49]
alginate and methylcellulose dissolved in human fresh levels of albumin, MRP2, and CD31, and cytochrome
frozen plasma that endowed the core–shell constructs P450 enzyme activity; this emphasizes the importance of
with excellent printability and supportive bioactivity. architectural cellular positioning to promote hepatic and
Thus, core–shell printing allowed for establishing an vascular function. In addition to physiological liver tissue
in vitro triple culture model of the hepatic sinusoid to modeling, 3D-bioprinted liver tissue can be applied in
investigate cell behavior in response to tailoring the 3D disease modeling. For example, to develop a liver fibrosis
microenvironment. Interactions between the different model, coordinated patterning was used to generate
cell types in the triple culture model were characterized organized hepatic layers composed of hepatocytes,
in terms of the bioprinted HepG2 functionality (i.e., activated stellate cells, and ECs . Through the multi-
[50]
improved albumin secretion in the presence of HUVEC, material printing process, each non-parenchymal hepatic
the necessity of supportive fibroblasts for angiogenesis, cell type was accurately localized into a multi-layered
and the competing effect of HepG2). In a different study, construct using gelatin and liver dECM bioinks. While
[49]
Kang et al. proposed the pre-set extrusion bioprinting hepatocytes-laden liver dECM bioink was exploited to
in which different bioinks were simultaneously extruded provide a 3D liver-specific microenvironment, gelatin
through a precursor cartridge to create multi-material bioink was used to deliver ECs or activated stellate cells
patterning according to the pre-defined configuration for monolayer formation. Overall, a unique multi-layered
without structural deformation (Figure 2D). Using this liver fibrosis model was reproduced to mimic cellular
approach, a heterogeneous, multi-cellular, and multi- distribution of human liver fibrosis. The 3D-bioprinted
material construct was bioprinted to form a hepatic lobule- liver fibrosis-on-a-chip platform exhibited hallmarks of
like structure with a pre-set arrangement of hepatocytes fibrotic remodeling, including collagen accumulation, cell
and ECs with a central lumen. The liver construct with apoptosis, and reduced liver function, mainly owing to the
biomimetic heterocellular localization was found to presence of the stellate cell population. For drug screening
enhance hepatic maturation compared with mix-printed application, drugs for downregulating hepatic stellate cell
constructs lacking geometric structure, as evidenced activation were treated on the 3D-bioprinted liver fibrosis
by the enriched secretion of urea and albumin, protein
Volume 9 Issue 5 (2023) 24 https://doi.org/10.18063/ijb.748