Page 33 - IJB-9-5
P. 33
International Journal of Bioprinting 3D bioprinted vascularized tissue models
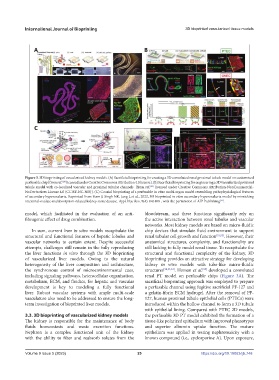
Figure 3. 3D bioprinting of vascularized kidney models. (A) Sacrificial bioprinting for creating a 3D convoluted renal proximal tubule model on customized
perfusable chip [from ref. licensed under Creative Commons Attribution 4.0 license]. (B) Sacrificial bioprinting for engineering a 3D vascularized proximal
[28]
tubule model with co-localized vascular and proximal tubular channels [from ref. licensed under Creative Commons Attribution-NonCommercial-
[53]
NoDerivatives License 4.0 (CC BY-NC-ND)]. (C) Coaxial bioprinting of a perfusable in vitro multi-organ model resembling pathophysiological features
of secondary hyperoxaluria. Reprinted from Yoon J, Singh NK, Jang J, et al., 2022, 3D bioprinted in vitro secondary hyperoxaluria model by mimicking
intestinal-oxalate-malabsorption-related kidney stone disease. Appl Phys Rev, 9(4): 041408 , with the permission of AIP Publishing .
[55]
model, which facilitated in the evaluation of an anti- bloodstream, and these functions significantly rely on
fibrogenic effect of drug combination. the active interaction between renal tubules and vascular
networks. Most kidney models are based on micro-fluidic
In sum, current liver in vitro models recapitulate the chip devices that simulate fluid environment to support
structural and functional features of hepatic lobules and renal tubular cell growth and function [51,52] . However, their
vascular networks to certain extent. Despite successful anatomical structures, complexity, and functionality are
attempts, challenges still remain in the fully reproducing still lacking to fully model renal tissue. To recapitulate the
the liver functions in vitro through the 3D bioprinting structural and functional complexity of the kidney, 3D
of vascularized liver models. Owing to the natural bioprinting provides an attractive strategy for developing
heterogeneity of the liver composition and architecture, kidney in vitro models with tube-like micro-fluidic
the synchronous control of microenvironmental cues, structures [28,53,54] . Homan et al. developed a convoluted
[28]
including signaling pathways, heterocellular organization, renal PT model on perfusable chips (Figure 3A). The
metabolism, ECM, and fluidics, for hepatic and vascular sacrificial bioprinting approach was employed to prepare
development is key to modeling a fully functional a perfusable channel using fugitive sacrificial PF-127 and
liver. Robust vascular systems with ample multi-scale a gelatin-fibrin ECM hydrogel. After the removal of PF-
vasculature also need to be addressed to ensure the long- 127, human proximal tubule epithelial cells (PTECs) were
term investigation of bioprinted liver models. introduced within the hollow channel to form a 3D tubule
with epithelial lining. Compared with PTEC 2D models,
3.3. 3D bioprinting of vascularized kidney models the perfusable 3D PT model exhibited the formation of a
The kidney is responsible for the maintenance of body tissue-like polarized epithelium with improved phenotypes
fluids homeostasis and waste excretion functions. and superior albumin uptake function. The mature
Nephron is a complex functional unit of the kidney epithelium was applied in testing nephrotoxicity with a
with the ability to filter and reabsorb solutes from the known compound (i.e., cyclosporine A). Upon exposure,
Volume 9 Issue 5 (2023) 25 https://doi.org/10.18063/ijb.748