Page 256 - IJB-9-6
P. 256
International Journal of Bioprinting 3D-Printed GelMA biomaterials in cartilage repair
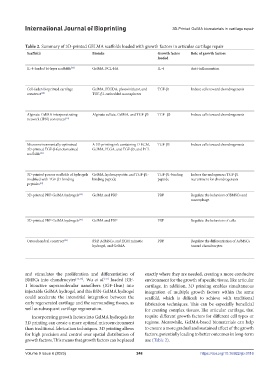
Table 2. Summary of 3D-printed GELMA scaffolds loaded with growth factors in articular cartilage repair
Scaffolds Bioinks Growth factor Role of growth factors Characteristics of scaffolds Results
loaded
In vitro In vivo
IL-4-loaded bi-layer scaffolds [44] GelMA, PCL-HA IL-4 Anti-inflammation • Multi-layers with different bio-inks and • Both layers supported cell adhesion and proliferation • New cartilage and subchondral
different functions • The upper layer relieved the inflammation of bone regeneration
• Upper: GelMA; lower: PCL-HA chondrocytes induced by IL-1b • Good mechanical strength similar
• The lower layer promoted osteogenesis to that of native cartilage
Cell-laden bioprinted cartilage GelMA, PEGDA, photoinitiator, and TGF-β1 Induce cells toward chondrogenesis • Fabricated via a core-shell electrospraying • Cells and nanospheres were evenly distributed • None
construct [38] TGF-β1-embedded nanospheres technique • Highest cell viability and proliferation on 5%/10%
• Modulus and swelling ratio could be adjusted (PEGDA/GelMA) hydrogel
by the addition of different PEGDA • Improved chondrogenic differentiation of
• Sustained release of TGF-β1 encapsulated MSCs.
Alginate-GelMA interpenetrating Alginate sulfate, GelMA, and TGF-β3 TGF- β3 Induce cells toward chondrogenesis • Maintained viscosity, shear-thinning and • Supported viability and robust chondrogenesis of • Supported chondrogenesis in vivo
network (IPN) constructs [39] thixotropic properties MSCs • Controlled release of TGF-β3
• High-fidelity bioprinting promoted cartilage-specific ECM
• Increased stiffness, and maintained resilience deposition
and toughness
• Sustained release of TGF- β3
Microenvironmentally optimized A 3D printing ink containing D-ECM, TGF-β3 Induce cells toward chondrogenesis • Microenvironment regulation • Directed endogenous stem/progenitor cell migration • Improved tissue repair outcomes in
3D-printed TGF-β-functionalized GelMA, PLGA, and TGF-β3, and PCL and differentiation the sheep animal model
scaffolds [40] • Guided more organized neotissue
formation
• Recapitulated the anisotropic
structure
3D-printed porous scaffolds of hydrogels GelMA, hydroxyapatite, and TGF-β1- TGF-β1-binding Induce the endogenous TGF-β1 • Multi-layers with different components and • Induced cartilage and osteogenic differentiation • Promoted osteochondral repair of
modified with TGF-β1-binding binding peptide peptide recruitment for chondrogenesis function rats
peptides [41] • Upper: GelMA, TGF-β1 binding peptide; • Recovered the animal gait behavior
lower: GelMA, hydroxyapatite
3D-printed PRP-GelMA hydrogels [49] GelMA and PRP PRP Regulate the behaviors of BMSCs and • Fabricated using the digital micro-mirror • Promoted proliferation, migration, and osteogenesis • More cartilage and subchondral
macrophage device (DMD) technique and chondrogenesis of BMSCs by 20% PRP/GelMA bone regeneration
• Promoted M2 polarization by 20% PRP/GelMA • More M2 macrophage infiltration
• Similar biological roles in BMSCs but less and less M1 macrophage
osteogenesis by 50% PRP/GelMA presentation
3D-printed PRP-GelMA hydrogels [50] GelMA and PRP PRP Regulate the behaviors of cells • Photoactivated PRP-based patient-specific • Long-term and constant rate growth factor release • Facilitated the proliferation and
bioink • Bioactivity protection of PRP differentiation of the ATDC5 cells
• Had the desired mechanical properties (low • Satisfactory mechanical characteristics
degradation rate and high mechanical strength)
Osteochondral construct [51] PRP, AdMSCs, and ECM mimetic PRP Regulate the differentiation of AdMSCs • Gradual printing of bio-inks • Induced glycosaminoglycan and calcium secretion, • None
hydrogel, and GelMA toward chondrocytes • Relatively low degradation rate and high mineralization, and ECM production
mechanical strength • Upregulated bone- and cartilage-unique genes
• Tissue-specific biomimetic structure
and stimulates the proliferation and differentiation of exactly where they are needed, creating a more conducive
BMSCs into chondrocytes [35,36] . Wu et al. loaded IGF- environment for the growth of specific tissue, like articular
[37]
1 bioactive supramolecular nanofibers (IGF-1bsn) into cartilage. In addition, 3D printing enables simultaneous
injectable GelMA hydrogel, and this BSN-GelMA hydrogel integration of multiple growth factors within the same
could accelerate the interstitial integration between the scaffold, which is difficult to achieve with traditional
early regenerated cartilage and the surrounding tissues, as fabrication techniques. This can be especially beneficial
well as subsequent cartilage regeneration. for creating complex tissues, like articular cartilage, that
Incorporating growth factors into GelMA hydrogels for require different growth factors for different cell types or
3D printing can create a more optimal microenvironment regions. Meanwhile, GelMA-based biomaterials can help
than traditional fabrication techniques. 3D printing allows to ensure a more gradual and sustained effect of the growth
for high precision and control over spatial distribution of factors, potentially leading to better outcomes in long-term
growth factors. This means that growth factors can be placed use (Table 2).
Volume 9 Issue 6 (2023) 248 https://doi.org/10.36922/ijb.0116