Page 59 - IMO-2-3
P. 59
Innovative Medicines & Omics Biocompatibility of nanomaterials
Magnesium, in particular, appears to play a supportive scaffold systems are increasingly being functionalized
role in modulating osteoblast responses and stimulating with biologically active molecules. Among the most
new bone matrix deposition. Its inclusion reflects a broader promising are BMP-2 and VEGF, which are well known for
trend toward ion-enhanced strategies for fine-tuning promoting osteogenesis and angiogenesis, respectively.
46
scaffold performance. As highlighted in Tables 1 and 2, These signaling molecules are often embedded within
nanomaterials used in these systems vary widely in their biodegradable carriers—such as PLGA microparticles—
biocompatibility and regenerative potential, emphasizing which facilitate controlled, localized release while
the importance of careful material selection and design preserving bioactivity over time. In parallel, gene delivery
optimization for translational success. strategies have gained significant traction. For example,
In pursuit of improved therapeutic outcomes, plasmid DNA encoding VEGF or BMP-2 has been
immobilized within CaP-based scaffolds, forming gene-
activated matrices that stimulate site-specific expression
of regenerative signals. Although these approaches have
demonstrated considerable potential, challenges remain—
particularly with maintaining vector stability during
scaffold fabrication, ensuring effective gene transfection,
and avoiding unintended off-target effects.
Additive manufacturing is also transforming the
landscape of scaffold development. In 2022, patient-
specific CaP-based craniofacial scaffolds achieved a
clinical success rate of over 95%, demonstrating both
feasibility and therapeutic promise. Technologies such
47
as fused deposition modeling and stereolithography are
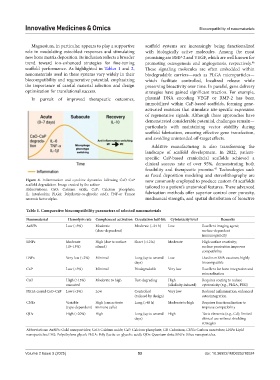
Figure 4. Inflammation and cytokine dynamics following CaO–CaP now commonly employed to produce custom-fit scaffolds
scaffold degradation. Image created by the author. tailored to a patient’s anatomical features. These advanced
Abbreviations: CaO: Calcium oxide; CaP: Calcium phosphate;
IL: Interleukin; PLGA: Poly(lactic-co-glycolic acid); TNF-α: Tumor fabrication methods offer superior control over porosity,
necrosis factor alpha. mechanical strength, and spatial distribution of bioactive
Table 1. Comparative biocompatibility parameters of selected nanomaterials
Nanomaterial Hemolysis rate Complement activation Circulation half‑life Cytotoxicity level Remarks
AuNPs Low (<5%) Moderate Moderate (~24 h) Low Excellent imaging agent;
(dose-dependent) surface-dependent
immunogenicity
SiNPs Moderate High (due to surface Short (<12 h) Moderate High surface reactivity;
(10–15%) silanol) surface passivation improves
compatibility
LNPs Very low (<2%) Minimal Long (up to several Low Used in mRNA vaccines; highly
days) biocompatible
CaP Low (<5%) Minimal Biodegradable Very low Excellent for bone integration and
mineralization
CaO High (>15%) Moderate to high Fast-degrading High Requires coating to reduce
uncoated (alkalinity-induced) cytotoxicity (e.g., PLGA, PEG)
PLGA-coated CaO–CaP Low (<3%) Low Controlled Very low Reduced inflammation; enhanced
(tailored by design) osteointegration
CNTs Variable High (can activate Long (>48 h) Moderate to high Requires functionalization to
(type-dependent) immune cells) improve compatibility
QDs High (>20%) High Long (up to several High Toxic elements (e.g., Cd); limited
days) clinical use without shielding
strategies
Abbreviations: AuNPs: Gold nanoparticles; CaO: Calcium oxide; CaP: Calcium phosphate; Cd: Cadmium; CNTs: Carbon nanotubes; LNPs: Lipid
nanoparticles; PEG: Polyethylene glycol; PLGA: Poly (lactic-co-glycolic acid); QDs: Quantum dots; SiNPs: Silica nanoparticles.
Volume 2 Issue 3 (2025) 53 doi: 10.36922/IMO025210024