Page 10 - ITPS-5-2
P. 10
4 INNOSC Theranostics and Pharmacological Sciences, 2022, Vol. 5, No. 2 Dhokne et al.
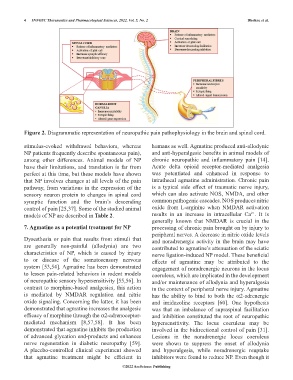
Figure 2. Diagrammatic representation of neuropathic pain pathophysiology in the brain and spinal cord.
stimulus-evoked withdrawal behaviors, whereas humans as well. Agmatine produced anti-allodynic
NP patients frequently describe spontaneous pain), and anti-hyperalgesic benefits in animal models of
among other differences. Animal models of NP chronic neuropathic and inflammatory pain [14].
have their limitations, and translation is far from Acute delta opioid receptor-mediated analgesia
perfect at this time, but these models have shown was potentiated and enhanced in response to
that NP involves changes at all levels of the pain intrathecal agmatine administration. Chronic pain
pathway, from variations in the expression of the is a typical side effect of traumatic nerve injury,
sensory neuron protein to changes in spinal cord which can also activate NOS, NMDA, and other
synaptic function and the brain’s descending common pathogenic cascades. NOS produces nitric
control of pain [25,37]. Some of the studied animal oxide from L-arginine when NMDAR activation
2+
models of NP are described in Table 2. results in an increase in intracellular Ca . It is
generally known that NMDAR is crucial in the
7. Agmatine as a potential treatment for NP processing of chronic pain brought on by injury to
peripheral nerves. A decrease in nitric oxide levels
Dysesthesia or pain that results from stimuli that and noradrenergic activity in the brain may have
are generally non-painful (allodynia) are two contributed to agmatine’s attenuation of the sciatic
characteristics of NP, which is caused by injury nerve ligation-induced NP model. These beneficial
to or disease of the somatosensory nervous effects of agmatine may be attributed to the
system [53,54]. Agmatine has been demonstrated engagement of noradrenergic neurons in the locus
to lessen pain-related behaviors in rodent models coeruleus, which are implicated in the development
of neuropathic sensory hypersensitivity [55,56]. In and/or maintenance of allodynia and hyperalgesia
contrast to morphine-based analgesics, this action in the context of peripheral nerve injury. Agmatine
is mediated by NMDAR regulation and nitric has the ability to bind to both the α2-adrenergic
oxide signaling. Concerning the latter, it has been and imidazoline receptors [60]. One hypothesis
demonstrated that agmatine increases the analgesic was that an imbalance of supraspinal facilitation
efficacy of morphine through the α2-adrenoceptor- and inhibition constituted the root of neuropathic
mediated mechanism [8,57,58]. It has been hypersensitivity. The locus coeruleus may be
demonstrated that agmatine inhibits the production involved in the bidirectional control of pain [31].
of advanced glycation end-products and enhances Lesions in the noradrenergic locus coeruleus
nerve regeneration in diabetic neuropathy [59]. were shown to suppress the onset of allodynia
A placebo-controlled clinical experiment showed and hyperalgesia, while noradrenergic reuptake
that agmatine treatment might be efficient in inhibitors were found to reduce NP. Even though it
©2022 AccScience Publishing