Page 45 - ITPS-6-2
P. 45
INNOSC Theranostics and
Pharmacological Sciences Genotoxicity of (4-fluorophenyl) thiazolidin-4-one
at position 2, 4, or 5. Depending on the position of the oxo
group or keto group, they are classified as 2-thiazolidinones,
4-thiazolidinones, and 5-thiazolidinones . The applications
[4]
of 4-thiazolidinone compounds are particularly diverse
and can be found in a variety of clinically employed drugs.
These compounds exhibit antitubercular , antimicrobial ,
[5,6]
[4]
anticonvulsant [7-9] , anti-inflammatory and analgesic , and
[10]
anti-cancer [11-13] properties. In addition, they have been reported
as cox-1 inhibitors , bacterial enzyme inhibitors, and non-
[14]
nucleoside inhibitors of HIV Type 1 reverse transcriptase [15-20] .
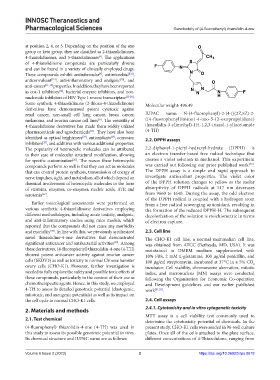
Some synthetic 4-thiazolidinone (2-thioxo-4-thiazolidinone) Molecular weight: 496.49
derivatives have demonstrated potent cytotoxic against
renal cancer, non-small cell lung cancer, breast cancer, IUPAC name: N-(4-fluorophenyl)-2-(4-(((2Z,5Z)-2-
melanoma, and ovarian cancer cell lines . The versatility of ((4-fluorophenyl)imino)-4-oxo-5-(2-oxopropylidene)
[21]
4-thiazolidinone derivatives has made them widely utilized thiazolidin-3-yl)methyl)-1H-1,2,3-triazol-1-yl)acetamide
[22]
pharmaceuticals and agrochemicals . They have also been (4-TH)
identified as optical brighteners , antioxidants , corrosion 2.2. DPPH assays
[24]
[23]
inhibitors , and additives with various additional properties.
[25]
The popularity of heterocyclic molecules can be attributed 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) is
to their ease of molecular structural modification, allowing an electron transfer-based free radical technique that
[26]
for specific customization . The reason these heterocyclic creates a violet solution in methanol. This experiment
compounds perform so well is that they can act as molecules was carried out following our prior published work [30] .
that can control protein synthesis, transmission of energy of The DPPH assay is a simple and rapid approach to
nerve impulses, sight, and metabolism, all of which depend on investigate antioxidant properties. The violet color
chemical involvement of heterocyclic molecules in the form of the DPPH solution changes to yellow as the molar
of vitamins, enzymes, co-enzymes, nucleic acids, ATP, and absorptivity of DPPH radicals at 517 nm decreases
serotonin . from 9660 to 1640. During the assay, the odd electron
[27]
of the DPPH radical is coupled with a hydrogen atom
Earlier toxicological assessments were performed on from a free radical scavenging antioxidant, resulting in
various synthetic 4-thiazolidinone derivatives employing the formation of the reduced DPPH-H. The subsequent
different methodologies, including acute toxicity, analgesic, decolorization of the solution is stoichiometric in terms
and anti-inflammatory studies using mice models, which of electron capture.
reported that the compounds did not cause any morbidity
and mortality . In line with this, we previously synthesized 2.3. Cell line
[28]
novel thiazolidine-4-one derivatives that demonstrated The CHO-K1 cell line, a normal mammalian cell line,
significant anticancer and antibacterial activities . Among was obtained from ATCC (Bethesda, MD, USA). It was
[29]
these derivatives, (4-fluorophenyl) thiazolidin-4-one (4-TH) maintained in DMEM medium supplemented with
showed potent anticancer activity against ovarian cancer 10% FBS, 2 mM L-glutamine, 100 µg/ml penicillin, and
cells (SKOV3) as well as toxicity to normal Chinese hamster 100 µg/ml streptomycin, incubated at 37°C in a 5% CO
2
ovary cells (CHO-K1). However, further investigation is incubator. Cell viability, chromosome aberration, mitotic
needed to fully explore the safety and possible toxic effects of index, and micronucleus (MN) assays were conducted
these compounds, particularly in the context of their use as following the Organisation for Economic Co-operation
chemotherapeutic agents. Hence, in this study, we employed and Development guidelines and our earlier published
4-TH to assess its detailed genotoxic potential (clastogenic, work [31,32] .
mitotoxic, and aneugenic potentials) as well as its impact on
the cell cycle in normal CHO-K1 cells. 2.4. Cell assays
2. Materials and methods 2.4.1. Cytotoxicity and in vitro cytogenetic toxicity
MTT assay is a cell viability test commonly used to
2.1. Test chemical
determine the cytotoxicity potential of chemicals. In the
(4-fluorophenyl) thiazolidin-4-one (4-TH) was used in present study, CHO-K1 cells were seeded in 96-well culture
this study to assess its possible genotoxic potential in vitro. plates. Once all of the cells attached to the plate surface,
Its chemical structure and IUPAC name are as follows: different concentrations of 4-Thiozolidone, ranging from
Volume 6 Issue 2 (2023) 2 https://doi.org/10.36922/itps.0618