Page 69 - ITPS-7-4
P. 69
INNOSC Theranostics and
Pharmacological Sciences Role of saroglitazar in MASH
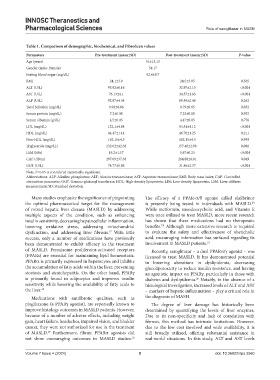
Table 1. Comparison of demographic, biochemical, and FibroScan values
Parameters Pre‑treatment (mean±SD) Post‑treatment (mean±SD) P‑value
Age (years) 51±13.13
Gender (male: Female) 34:17
Fasting blood sugar (mg/dL) 92.6±9.7
BMI 24.1±3.9 24.5±3.05 0.565
ALT (U/L) 93.82±6.16 32.97±2.15 <0.001
AST (U/L) 76.13±4.1 34.57±1.65 <0.001
ALP (U/L) 92.87±4.34 89.59±2.48 0.262
Total bilirubin (mg/dL) 0.90±0.06 0.79±0.05 0.032
Serum protein (mg/dL) 7.2±0.08 7.23±0.08 0.932
Serum albumin (g/dL) 4.5±0.05 4.47±0.05 0.776
LDL (mg/dL) 122.1±4.84 93.51±4.12 <0.001
HDL (mg/dL) 44.47±1.14 46.70±1.25 0.211
Non-HDL (mg/dL) 162.15±4.5 162.15±4.5 0.999
Triglyceride (mg/dL) 232.02±42.38 157.48±2.98 0.086
LSM (kPa) 16.2±1.57 5.67±0.23 <0.001
CAP (dB/m) 297.09±37.38 264.80±6.81 0.049
GGT (U/L) 74.77±5.86 31.86±2.37 <0.001
Note: P<0.05 is considered statistically significant.
Abbreviations: ALP: Alkaline phosphatase; ALT: Alanine transaminase; AST: Aspartate transaminase; BMI: Body mass index; CAP: Controlled
attenuation parameter; GGT: Gamma-glutamyl transferase; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; LSM: Liver stiffness
measurement; SD: Standard deviation.
Many studies emphasize the significance of pinpointing The efficacy of a PPAR-α/δ agonist called elafibrinor
the optimal pharmaceutical target for the management is presently being tested in individuals with MASLD.
20
of mixed hepatic liver disease (MASLD) by addressing While metformin, ursodeoxycholic acid, and Vitamin E
multiple aspects of the condition, such as enhancing were once utilized to treat MASLD, more recent research
insulin sensitivity, decreasing hepatocellular inflammation, has shown that these medications had no therapeutic
7,8
lowering oxidative stress, addressing mitochondrial benefits. Although more extensive research is required
dysfunction, and addressing liver fibrosis. With little to evaluate the safety and effectiveness of obeticholic
17
success, only a number of medications have previously acid, encouraging information has surfaced regarding its
been demonstrated to exhibit efficacy in the treatment involvement in MASLD patients. 21
of MASLD. Peroxisome proliferator-activated receptors Recently, saroglitazar – a dual PPARα/γ agonist – was
(PPARs) are essential for maintaining lipid homeostasis. licensed to treat MASLD. It has demonstrated potential
PPARα is primarily expressed in hepatocytes and inhibits in lowering alterations in dyslipidemia, decreasing
the accumulation of fatty acids within the liver, preventing glucolipotoxicity to reduce insulin resistance, and having
steatosis and steatohepatitis. On the other hand, PPARγ an agonistic impact on PPARγ, particularly in those with
is primarily found in adipocytes and improves insulin diabetes and dyslipidemia. Notably, in the absence of a
20
sensitivity while lowering the availability of fatty acids to histological investigation, increased levels of ALT and AST
the liver. 18 – markers of hepatic inflammation – play a critical role in
Medications with antifibrotic qualities, such as the diagnosis of MASH.
pioglitazone (a PPARγ agonist), are reportedly known to The degree of liver damage has historically been
improve histology outcomes in MASLD patients. However, determined by quantifying the levels of liver enzymes.
because of a number of adverse effects, including weight Due to its non-specificity and lack of correlation with
gain, heart failure, headaches, impaired vision, and bladder fibrosis, this method has intrinsic limitations. However,
cancer, they were not authorized for use in the treatment due to the low cost involved and wide availability, it is
of MASLD. Furthermore, fibrate PPARα agonists did still broadly utilized, offering substantial assistance in
19
not show encouraging outcomes in MASLD studies. real-world situations. In this study, ALT and AST levels
12
Volume 7 Issue 4 (2024) 5 doi: 10.36922/itps.3560