Page 11 - JCTR-11-2
P. 11
Journal of Clinical and
Translational Research US-mediated drug delivery
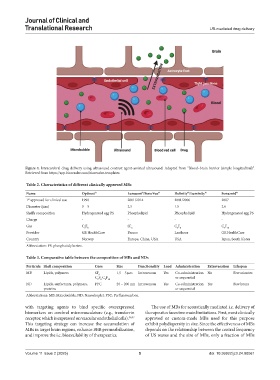
Figure 4. Intracerebral drug delivery using ultrasound contrast agent-assisted ultrasound. Adapted from “Blood–brain barrier (simple longitudinal)”.
Retrieved from https://app.biorender.com/biorender-templates.
Table 2. Characteristics of different clinically approved MBs
Name Optison ® Lumason /SonoVue ® Definity /Luminity ® Sonazoid ®
®
®
1 approved for clinical use 1998 2001/2014 2001/2006 2007
st
Diameter (µm) 3 – 5 2.5 1.5 2.6
Shell’s composition Hydrogenated egg PS Phospholipid Phospholipid Hydrogenated egg PS
Charge - - - -
Gas C F SF C F C F
3 8 6 3 8 4 10
Provider GE HealthCare Bracco Lantheus GE HealthCare
Country Norway Europe, China, USA USA Japan, South Korea
Abbreviation: PS: phosphatidylserine.
Table 3. Comparative table between the composition of MBs and NDs
Particule Shell composition Core Size Functionality Load Administration Extravasation Lifespan
MB Lipids, polymers SF 6 1.5 – 5 µm Intravenous Yes Co-administration No Few minutes
C F C F or sequential
3 8 4 10
ND Lipids, surfactants, polymers, PFC 20 – 200 nm Intravenous Yes Co-administration Yes Few hours
proteins or sequential
Abbreviations: MB: Microbubble; ND: Nanodroplet: PFC: Perfluorocarbon.
with targeting agents to bind specific overexpressed The use of MBs for acoustically mediated i.c. delivery of
biomarkers on cerebral microvasculature (e.g., transferrin therapeutics faces two main limitations. First, most clinically
receptor, which is expressed on vascular endothelial cells). 36,37 approved or custom-made MBs used for this purpose
This targeting strategy can increase the accumulation of exhibit polydispersity in size. Since the effectiveness of MBs
MBs in target brain regions, enhance BBB permeabilization, depends on the relationship between the central frequency
and improve the i.c. bioavailability of therapeutics. of US waves and the size of MBs, only a fraction of MBs
Volume 11 Issue 2 (2025) 5 doi: 10.36922/jctr.24.00061