Page 100 - JCTR-11-3
P. 100
Journal of Clinical and
Translational Research Household arsenic and bladder cancer
incubated with iAs for 48 h identified a lower genotoxic
threshold of 1 μM using the same assay, though this level
30
was not observed in our patient cohort. However, iAs also
exert non-genotoxic effects such as oxidative stress and
inhibition of DNA repair, 31,32 and the thresholds for these
effects remain poorly defined in primary urothelial cells.
Arsenic was detected in all indoor dust samples, with
significantly higher concentrations observed in UCC
households compared to controls. In addition, dust arsenic
levels were positively correlated with urinary arsenic
species across all patients. This suggests that indoor dust
may be a relevant source of urinary arsenic exposure in this
primarily non-smoking population. Arsenic accumulates
in indoor dust through airborne particulate matter
originating from sources of outdoor soil and roadway dust
33
and may be absorbed through inhalation or unintentional
ingestion. Although our study did not find an association
34
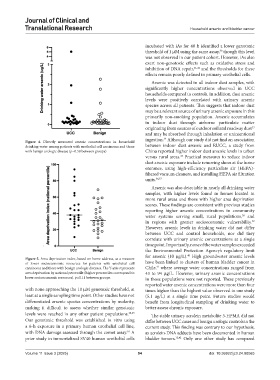
Figure 4. Directly measured arsenic concentrations in household
drinking water among patients with urothelial cell carcinoma and those between indoor dust arsenic and RUCC, a study from
with benign urologic disease (p=0.58 between groups) China reported higher indoor dust arsenic levels in urban
35
versus rural areas. Practical measures to reduce indoor
dust arsenic exposure include removing shoes at the home
entrance, using high-efficiency particulate air (HEPA)-
filtered vacuum cleaners, and installing HEPA air filtration
units. 36,37
Arsenic was also detectable in nearly all drinking water
samples, with higher levels found in homes located in
more rural areas and those with higher area deprivation
scores. These findings are consistent with previous studies
reporting higher arsenic concentrations in community
water systems serving small, rural populations, and
38
in regions with greater socioeconomic vulnerability.
39
However, arsenic levels in drinking water did not differ
between UCC and control households, nor did they
correlate with urinary arsenic concentrations at a single
time point. Importantly, none of the water samples exceeded
the Environmental Protection Agency’s regulatory limit
for arsenic (10 μg/L). High groundwater arsenic levels
40
Figure 5. Area deprivation index, based on home address, as a measure
of lower socioeconomic resources for patients with urothelial cell have been linked to clusters of human bladder cancer in
41
carcinoma and those with benign urologic diseases. The Y-axis represents Chile, where average water concentrations ranged from
area deprivation by national percentile (higher percentiles correspond to 43 to 94 μg/L. However, urinary arsenic concentrations
lower socioeconomic resources). p=0.11 between groups. in those populations were not reported. These previously
reported water arsenic concentrations were more than four
with none approaching the 10 μM genotoxic threshold, at times higher than the highest value observed in our study
least at a single sampling time point. Other studies have not (5.1 ug/L) at a single time point. Future studies would
differentiated arsenic species concentrations by molarity, benefit from longitudinal sampling of drinking water to
making it difficult to assess whether similar genotoxic better assess chronic exposure.
levels were reached in any other patient populations. 28,29 The stable urinary acrolein metabolite 3-HPMA did not
Our genotoxic threshold was established in vitro using differ between UCC cases and benign urologic controls in the
a 6-h exposure in a primary human urothelial cell line, current study. This finding was contrary to our hypothesis,
with DNA damage assessed through the comet assay. A as acrolein-DNA adducts have been documented in human
18
prior study in immortalized SV40 human urothelial cells bladder tumors. 12,42 Only one other study has compared
Volume 11 Issue 3 (2025) 94 doi: 10.36922/jctr.24.00065