Page 13 - JCTR-11-4
P. 13
Journal of Clinical and
Translational Research Hidden cancer after VTE
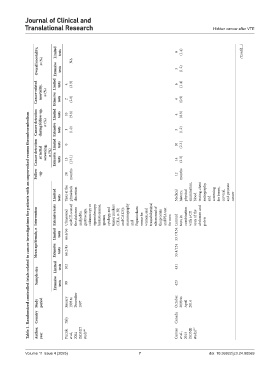
Overall mortality, n (%) Limited Extensive tests tests NA 6 5 (1.4) (1.2) (Cont’d...)
Cancer‑related mortality, n (%) Limited Extensive tests tests 4 2 (3.9) (2.0) 6 4 (1.4) (0.9)
Cancer detection during follow‑up, n (%) Limited Extensive tests tests 10 1 (9.8) (1.0) 4 5 (0.9) (1.2)
Table 1. Randomized controlled trials related to cancer investigations for patients with an unprovoked venous thromboembolism
Cancer detection at initial screening, n (%) Limited Extensive tests tests 0 13 (13.1) 10 14 (2.3.) (3.3)
Follow ‑up 24 months 12 months
Limited tests Tests at the physician’s discretion Medical history, physical examination, blood testing, chest radiography, and screening for breast, cervical, and prostate cancer
Intervention Extensive tests Ultrasound and CT-scan of the abdomen and pelvis, gastroscopy, colonoscopy or sigmoidoscopy, barium enema, sputum cytology, and tumor markers (CEA, α‐FP , and CA125); mammography and Papanicolaou smear for women, and transabdominal ultrasound of the prostate and PSA test for men Limited tests in combination with a CT scan of the abdomen and pelvis
Mean age/female, n Limited Extensive tests tests 66.6/56 66.2/45 53.7/154 53.4/124
Limited tests 102 431
Sample size Extensive tests 99 423
Study period January 1993 to December 1997 October 2008 to April 2014
Country Italy Canada
Author, year Piccioli et al., 2004 (SOMIT study 16 Carrier et al., 2015 (SOME study) 17
Volume 11 Issue 4 (2025) 7 doi: 10.36922/jctr.24.00069