Page 18 - JCTR-11-4
P. 18
Journal of Clinical and
Translational Research Hidden cancer after VTE
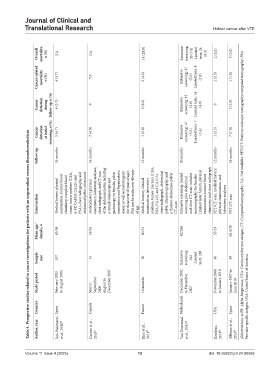
Overall mortality, n (%) NA NA 14 (28.0) Extensive screening: 26 (7.6) Limited tests: 24 (8.3) 2 (5.0) 3 (3.0)
Cancer‑related mortality, n (%) 4 (3.7) NA 2 (4.0) Extensive screening: 17 (5.0) Limited tests: 8 (2.8) 1 (2.5) 1 (1.0)
Cancer detection during follow‑up, n (%) 4 (3.7) 0 3 (6.0) Extensive screening: 13 (3.8) Limited tests: 14 (4.9) 0 2 (2.0)
Cancer detection at initial screening, n (%) 5 (4.7) 2 (4.0) 1 (2.0) Extensive screening: 12 (3.5) Limited tests: 7 (2.4) 1 (2.5) 7 (7.0)
Table 4. Prospective studies related to cancer investigations for patients with an unprovoked venous thromboembolism
Follow‑up 24 months 24 months 12 months 30 months 12 months 24 months
Intervention Medical history, physical examination, routine blood chemistry, complete blood count, tumor markers (CEA, α‐FP , CA19.9, CA125, and PSA), chest radiography, and abdominal/pelvic ultrasound Medical history, physical examination, laboratory analyses, chest radiograph, and CT scan of the abdomen/pelvis, including a virtual colonoscopy and gastroscopy; in females, pelvic examination and Papanicolaou smear, as well as mammogram
Mean age/ female, n 63/56 55/35 80/31 62/246 55/19 68.4/38
Sample size 107 51 50 Extensive screening: 342 Limited tests: 288 40 99
Study period February 2003 to August 2004 May to September 2006 August to December 2007 18 months December 2002 to December 2007 November 2008 to January 2010 January 2007 to June 2010
Country Spain Canada France Netherlands USA Spain Prostate‐specific antigen; USA: United States of America.
Author, year Jara-Palomares et al., 2010 20 Carrier et al., 2010 21 Rieu et al., 2011 22 Van Doormaal et al., 2011 23 Rondina, 2011 24 Alfonso et al., 2013 25
Volume 11 Issue 4 (2025) 12 doi: 10.36922/jctr.24.00069