Page 21 - JCTR-9-6
P. 21
Miyake et al. | Journal of Clinical and Translational Research 2023; 9(6): 381-391 385
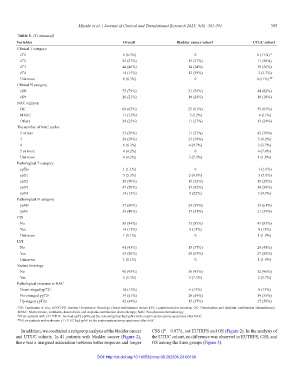
Table 1. (Continued)
Variables Overall Bladder cancer cohort UTUC cohort
Clinical T category
cT1 6 (6.3%) 0 6 (11%) #
cT2 25 (27%) 15 (37%) 11 (20%)
cT3 44 (46%) 14 (34%) 30 (56%)
cT4 14 (15%) 12 (29%) 2 (3.7%)
Unknown 6 (6.3%) 0 6 (11%) ##
Clinical N category
cN0 75 (79%) 31 (76%) 44 (82%)
cN+ 20 (21%) 10 (24%) 10 (18%)
NAC regimen
GC 60 (63%) 25 (61%) 35 (65%)
MVAC 11 (12%) 5 (12%) 6 (11%)
Others 24 (25%) 11 (27%) 13 (24%)
The number of NAC cycles
2 or less 53 (56%) 11 (27%) 42 (78%)
3 28 (29%) 23 (56%) 5 (9.2%)
4 6 (6.3%) 4 (9.7%) 2 (3.7%)
5 or more 4 (4.2%) 0 4 (7.4%)
Unknown 4 (4.2%) 3 (7.3%) 1 (1.8%)
Pathological T category
ypTis 1 (1.1%) 0 1 (1.9%)
ypT1 5 (5.3%) 2 (4.9%) 3 (5.6%)
ypT2 28 (30%) 13 (32%) 15 (28%)
ypT3 47 (50%) 17 (42%) 30 (56%)
ypT4 14 (15%) 9 (22%) 5 (9.3%)
Pathological N category
ypN0 57 (60%) 24 (59%) 33 (61%)
ypN+ 38 (40%) 17 (41%) 21 (39%)
CIS
No 80 (84%) 35 (85%) 45 (83%)
Yes 14 (15%) 6 (15%) 8 (15%)
Unknown 1 (1.1%) 0 1 (1.9%)
LVI
No 41 (43%) 15 (37%) 26 (48%)
Yes 53 (56%) 26 (63%) 27 (50%)
Unknown 1 (1.1%) 0 1 (1.9%)
Variant histology
No 90 (95%) 38 (93%) 52 (96%)
Yes 5 (5.3%) 3 (7.3%) 2 (3.7%)
Pathological response to NAC
Down-staged ypT2≤ 14 (15%) 6 (15%) 8 (15%)
No-changed ypT2≤ 39 (41%) 20 (49%) 19 (35%)
Up-staged ypT2≤ 42 (44%) 15 (37%) 27 (50%)
CIS: Carcinoma in situ; ECOG-PS: Eastern Cooperative Oncology Group-performance status; LVI: Lymphovascular invasion; GC: Gemcitabine and cisplatin combination chemotherapy;
MVAC: Methotrexate, vinblastin, doxorubicin, and cisplatin combination chemotherapy; NAC: Neoadjuvant chemotherapy.
# Of six patients with cT1 UTUC, two had ypT2 ypN0 and the remaining four had ypN+ in the nephroureterectomy specimens after NAC;
## All six patients with unknown cT UTUC had ypN+ in the nephroureterectomy specimens after NAC
In addition, we conducted a subgroup analysis of the bladder cancer CSS (P = 0.073), not EUTRFS and OS (Figure 2). In the analysis of
and UTUC cohorts. In 41 patients with bladder cancer (Figure 2), the UTUC cohort, no difference was observed in EUTRFS, CSS, and
there was a marginal association between better response and longer OS among the three groups (Figure 3).
DOI: http://dx.doi.org/10.18053/jctres.09.202306.23-00106