Page 65 - JCTR-9-6
P. 65
Malakar et al. | Journal of Clinical and Translational Research 2023; 9(6): 423-432 429
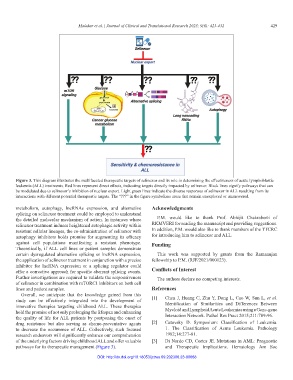
Figure 3. This diagram illustrates the multifaceted therapeutic targets of selinexor and its role in determining the effectiveness of acute lymphoblastic
leukemia (ALL) treatments. Red lines represent direct effects, indicating targets directly impacted by selinexor. Black lines signify pathways that can
be modulated due to selinexor’s inhibition of nuclear export. Light green lines indicate the diverse responses of selinexor in ALL resulting from its
interactions with different potential therapeutic targets. The “???” in the figure symbolizes areas that remain unexplored or unanswered.
metabolism, autophagy, lncRNAs expression, and alternative Acknowledgments
splicing on selinexor treatment could be employed to understand
the detailed molecular mechanism of action. In instances where P.M. would like to thank Prof. Abhijit Chakraborti of
selinexor treatment induces heightened autophagic activity within RKMVERI for reading the manuscript and providing suggestions.
resistant cellular lineages, the co-administration of selinexor with In addition, P.M. would also like to thank members of the TTCRC
autophagy inhibitors holds promise for augmenting its efficacy for introducing him to selinexor and ALL.
against cell populations manifesting a resistant phenotype. Funding
Theoretically, if ALL cell lines or patient samples demonstrate
certain dysregulated alternative splicing or lncRNA expression, This work was supported by grants from the Ramanujan
the application of selinexor treatment in conjunction with a precise fellowship to P.M. (RJF/2021/000123).
inhibitor for lncRNA expression or a splicing regulator could
offer a corrective approach for specific aberrant splicing events. Conflicts of Interest
Further investigations are required to validate the responsiveness The authors declare no competing interests.
of selinexor in combination with mTORC1 inhibitors on both cell
lines and patient samples. References
Overall, we anticipate that the knowledge gained from this
study can be effectively integrated into the development of [1] Chen J, Huang C, Zhu Y, Dong L, Cao W, Sun L, et al.
Identification of Similarities and Differences Between
innovative therapies targeting childhood ALL. These therapies
hold the promise of not only prolonging the lifespan and enhancing Myeloid and Lymphoid Acute Leukemias using a Gene-gene
the quality of life for ALL patients by postponing the onset of Interaction Network. Pathol Res Pract 2015;211:789-96.
drug resistance but also serving as chemo-preventative agents [2] Catovsky D. Symposium: Classification of Leukemia.
to decrease the occurrence of ALL. Collectively, such focused 1. The Classification of Acute Leukemia. Pathology
research endeavors will significantly enhance our comprehension 1982;14:277-81.
of the underlying factors driving childhood ALL and offer valuable [3] Di Nardo CD, Cortes JE. Mutations in AML: Prognostic
pathways for its therapeutic management (Figure 3). and Therapeutic Implications. Hematology Am Soc
DOI: http://dx.doi.org/10.18053/jctres.09.202306.23-00088