Page 61 - JCTR-9-6
P. 61
Malakar et al. | Journal of Clinical and Translational Research 2023; 9(6): 423-432 425
Table 1. Clinical trial studies of selinexor alone or in combination with chemotherapeutic drugs in AML
Drugs Type of leukemia Phases Outcome References
Selinexor AML Phase I Selinexor is safe as a monotherapy in patients with relapsed or [17]
refractory AML
Selinexor + Venetoclax AML Phase I This combination is a safe regimen for AML patients NCT04898894
Selinexor + Daunorubicin + Cytarabine AML Phase I This combination is a safe regimen for newly diagnosed poor-risk [18]
AML patients
Selinexor + Mitoxantrone (M) + AML Phase I Selinexor plus MEC is a feasible treatment for patients with R/R AML NCT02299518
Etoposide (E) + Cytarabine (C )
Selinexor + Cytarabine + Idarubicin AML Phase II Selinexor, cytarabine, and idarubicin result in a high remission rate in [19]
patients with R/R AML
AML: Acute myeloid leukemia
Table 2. Preclinical studies of selinexor alone or in combination with chemotherapeutic drugs in various cancers and their altered pathways
Drugs In vitro/In vivo studies Altered pathways References
Selinexor AML cell line Downregulation of mTOR signaling; regulate p53 pathway [13]
Selinexor + Dexamethasone Multiple myeloma cell line and Suppress mTORC1 signaling and inhibits tumor growth in [20]
multiple myeloma mice models both in vitro and in vivo studies
Selinexor + Azacitidine AML cell line Inhibit XPO1/eIF4E/c-MYC signaling [21]
Selinexor Gall bladder cancer cell line Autophagy-dependent apoptosis by activating the p53/ [22]
and mice models mTOR pathway
AML: Acute myeloid leukemia; mTOR: Mammalian target of rapamycin
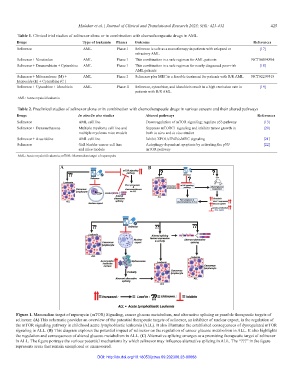
A B
C
Figure 1. Mammalian target of rapamycin (mTOR) Signaling, cancer glucose metabolism, and alternative splicing as possible therapeutic targets of
selinexor. (A) This schematic provides an overview of the potential therapeutic targets of selinexor, an inhibitor of nuclear export, in the regulation of
the mTOR signaling pathway in childhood acute lymphoblastic leukemia (ALL). It also illustrates the established consequences of dysregulated mTOR
signaling in ALL. (B) This diagram explores the potential impact of selinexor on the regulation of cancer glucose metabolism in ALL. It also highlights
the regulation and consequences of altered glucose metabolism in ALL. (C) Alternative splicing emerges as a promising therapeutic target of selinexor
in ALL. The figure portrays the various potential mechanisms by which selinexor may influence alternative splicing in ALL. The “???” in the figure
represents areas that remain unexplored or unanswered.
DOI: http://dx.doi.org/10.18053/jctres.09.202306.23-00088