Page 23 - MI-1-2
P. 23
Microbes & Immunity Homologous versus heterologous COVID-19 vaccines
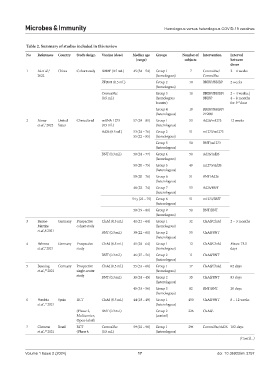
Table 2. Summary of studies included in this review
No References Country Study design Vaccine (dose) Median age Groups Number of Intervention Interval
(range) subjects between
doses
1 Ai et al., 6 China Cohort study BBIBP (0.5 mL) 45 (34 – 54) Group 1 7 CoronaVac/ 2 – 4 weeks
2022 (homologous) CoronaVac
ZF2001 (0.5 mL) Group 2 10 BBIBP/BBIBP 2 weeks
(homologous)
CoronaVac Group 3 10 BBIBP/BBIBP/ 2 – 4 weeks; |
(0.5 mL) (homologous BBIBP 4 – 8 months
booster) for 3 dose
rd
Group 4 10 BBIBP/BBIBP/
(heterologous) ZF2001
2 Atmar United Clinical trial mRNA-1273 57 (24 – 81) Group 1 53 Ad26/m1273 12 weeks
7
et al., 2022 States (0.5 mL) (heterologous)
Ad26 (0.5 mL) 53 (24 – 76) Group 2 51 m1273/m1273
55 (22 – 85) (homologous)
Group 3 50 BNT/m1273
(heterologous)
BNT (0.3 mL) 50 (24 – 77) Group 4 50 Ad26/Ad26
(homologous)
50 (20 – 75) Group 5 49 m1273/Ad26
(heterologous)
50 (20 – 76) Group 6 51 BNT/Ad26
(heterologous)
48 (22 – 74) Group 7 53 Ad26/BNT
(heterologous)
54 y (23 – 75) Group 8 51 m1273/BNT
(heterologous)
50 (19 – 80) Group 9 50 BNT/BNT
(homologous)
3 Barros- Germany Prospective ChAd (0.5 mL) 41 (21 – 64) Group 1 32 ChAd/ChAd 2 – 3 months
Martins cohort study (homologous)
et al.,8 2021 BNT (0.3 mL) 39 (22 – 61) Group 2 55 ChAd/BNT
(heterologous)
4 Behrens Germany Prospective ChAd (0.5 mL) 41 (24 – 64) Group 1 12 ChAd/ChAd Mean: 73.5
9
et al., 2021 study (homologous) days
BNT (0.3 mL) 46 (27 – 56) Group 2 11 ChAd/BNT
(heterologous)
5 Benning Germany Prospective ChAd (0.5 mL) 55 (33 – 60) Group 1 17 ChAd/ChAd 82 days
et al., 2021 single-center (homologous)
10
study BNT (0.3 mL) 30 (24 – 45) Group 2 35 ChAd/BNT 83 days
(heterologous)
45 (33 – 56) Group 3 82 BNT/BNT 20 days
(homologous)
6 Borobia Spain RCT ChAd (0.5 mL) 44 (18 – 49) Group 1 450 ChAd/BNT 8 – 12 weeks
11
et al., 2021 (heterologous)
(Phase 2, BNT (0.3 mL) Group 2 226 ChAd/-
Multicenter, (control)
Open-label)
7 Clemens Brazil RCT CoronaVac 59 (22 – 98) Group 1 294 CoronaVac/Ad26 182 days
et al., 2021 (Phase 4, (0.5 mL) (heterologous)
12
(Cont’d...)
Volume 1 Issue 2 (2024) 17 doi: 10.36922/mi.3757