Page 28 - MI-1-2
P. 28
Microbes & Immunity Homologous versus heterologous COVID-19 vaccines
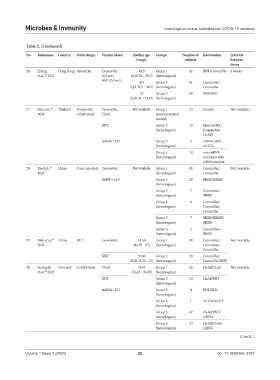
Table 2. (Continued)
No References Country Study design Vaccine (dose) Median age Groups Number of Intervention Interval
(range) subjects between
doses
26 Zhang Hong Kong Open trial CoronaVac 44.5 Group 1 42 BNT/CoronaVac 4 weeks
et al., 2022 (0.5 mL) (IQR 36 – 50.5) (heterologous)
31
BNT (0.3 mL) 49 ( Group 2 41 CoronaVac/
IQR 39.5 – 54.5) (homologous) CoronaVac
47 Group 3 40 BNT/BNT
(IQR 33 – 51.75) (homologous)
27 Sila et al., Thailand Prospective CoronaVac Not available Group 1 23 Control Not available
32
2024 cohort study ChAd (non-vaccinated
control)
BNT Group 2 14 Non-mRNA
(homologous) (CoronaVac,
ChAd)
mRNA-1273 Group 3 5 mRNA (BNT,
(homologous) m1273)
Group 4 10 non-mRNA
(heterologous) combined with
mRNA vaccine
28 Xu et al., China Case-case study CoronaVac Not available Group 1 40 CoronaVac/ Not available
33
2023 (homologous) CoronaVac
BBIBP-CorV Group 2 20 BBIBP/BBIBP
(homologous)
Group 3 7 CoronaVac/
(heterologous) BBIBP
Group 4 8 CoronaVac/
(homologous) CoronaVac/
CoronaVac
Group 5 7 BBIBP/BBIBP/
(homologous) BBIBP
Group 6 2 CoronaVac+
(heterologous) BBIBP
29 Mok et al., China RCT CoronaVac 51.50 Group 1 40 CoronaVac/ Not available
34
2022 (44.25 – 57) (homologous) CoronaVac/
CoronaVac
BNT 50.00 Group 2 40 CoronaVac/
(IQR 45.25 – 57) (heterologous) CoronaVac/BNT
30 Gerhards Germany Cohort study ChAd 39.64 Group 1 26 ChAd/ChAd Not available
et al., 2023 (24.83 – 54.45) (homologous)
35
BNT Group 2 53 ChAd/BNT
(heterologous)
mRNA-1273 Group 3 4 BNT/BNT
(homologous)
Group 4 1 m1273/m1273
(homologous)
Group 5 47 ChAd/BNT/
(homologous) mRNA
Group 6 24 ChAd/ChAd/
(heterologous) mRNA
(Cont’d...)
Volume 1 Issue 2 (2024) 22 doi: 10.36922/mi.3757