Page 25 - MI-1-2
P. 25
Microbes & Immunity Homologous versus heterologous COVID-19 vaccines
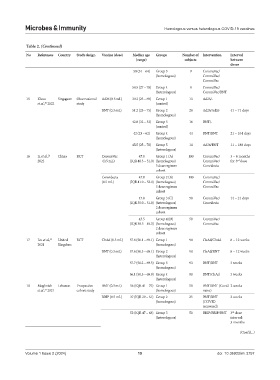
Table 2. (Continued)
No References Country Study design Vaccine (dose) Median age Groups Number of Intervention Interval
(range) subjects between
doses
58 (31 – 64) Group 3 9 CoronaVac/
(homologous) CoronaVac/
CoronaVac
58.5 (27 – 70) Group 4 8 CoronaVac/
(heterologous) CoronaVac/BNT
15 Khoo Singapore Observational Ad26 (0.5 mL) 39.2 (25 – 69) Group 1 13 Ad26/-
20
et al., 2022 study (control)
BNT (0.3 mL) 51.2 (25 – 75) Group 2 28 Ad26/Ad26 41 – 71 days
(homologous)
42.8 (32 – 53) Group 3 16 BNT/-
(control)
42 (23 – 62) Group 4 44 BNT/BNT 21 – 104 days
(homologous)
45.5 (25 – 70) Group 5 14 Ad26/BNT 11 – 180 days
(heterologous)
21
16 Li et al., China RCT CoronaVac 47.0 Group 1 (A) 100 CoronaVac/ 3 – 6 months
2022 (0.5 mL) (IQR 40.3 – 51.0) (homologous) CoronaVac/ for 3 dose
rd
3 dose regimen Convidecia
cohort
Convidecia 47.0 Group 2 (B) 100 CoronaVac/
(0.5 mL) (IQR 41.0 – 52.0) (homologous) CoronaVac/
3 dose regimen CoronaVac
cohort
47.0 Group 3 (C) 50 CoronaVac/ 14 – 21 days
(IQR 35.0 – 51.0) (heterologous) Convidecia
2 dose regimen
cohort
43.5 Group 4 (D) 50 CoronaVac/
(IQR 38.5 – 49.3) (homologous) CoronaVac
2 dose regimen
cohort
17 Liu et al., United RCT ChAd (0.5 mL) 57.6 (50.1 – 69.1) Group 1 90 ChAd/ChAd 8 – 12 weeks
22
2021 Kingdom (homologous)
BNT (0.3 mL) 57.6 (50.3 – 68.1) Group 2 90 ChAd/BNT 8 – 12 weeks
(heterologous)
57.7 (50.2 – 69.3) Group 3 93 BNT/BNT 3 weeks
(homologous)
56.1 (50.5 – 68.9) Group 4 90 BNT/ChAd 3 weeks
(heterologous)
18 Moghnieh Lebanon Prospective BNT (0.3 mL) 56 (IQR 41 – 75) Group 1 50 BNT/BNT (Covid 2 weeks
23
et al., 2021 cohort study (homologous) naïve)
BBIP (0.5 mL) 37 (IQR 29 – 61) Group 2 25 BNT/BNT 2 weeks
(homologous) (COVID
recovered)
52 (IQR 47 – 63) Group 3 50 BBIP/BBIP/BNT 3 dose
rd
(heterologous) interval:
3 months
(Cont’d...)
Volume 1 Issue 2 (2024) 19 doi: 10.36922/mi.3757