Page 26 - MI-1-2
P. 26
Microbes & Immunity Homologous versus heterologous COVID-19 vaccines
Table 2. (Continued)
No References Country Study design Vaccine (dose) Median age Groups Number of Intervention Interval
(range) subjects between
doses
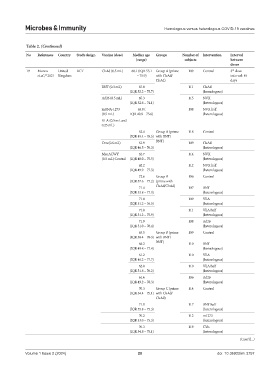
19 Munro United RCT ChAd (0.5 mL) 68.1 (IQR 55.1 Group A (prime 109 Control 3 dose
rd
et al., 2021 Kingdom – 75.9) with ChAd/ interval: 84
24
ChAd) days
BNT (0.3 mL) 67.8 111 ChAd
(IQR 52.2 – 75.7) (homologous)
Ad26 (0.5 mL) 65.3 115 NVX
(IQR 52.6 – 74.1) (heterologous)
mRNA-1273 65.8 ( 108 NVX half
(0.5 mL) IQR 49.9 – 75.6) (heterologous)
VLA (0.5 mL and
0.25 mL)
62.4 Group A (prime 118 Control
(IQR 49.4 – 78.5) with BNT/
Cvn (0.6 mL) 61.9 BNT) 109 ChAd
(IQR 46.5 – 76.3) (heterologous)
MenACWY 62.7 114 NVX
(0.5 mL) Control (IQR 48.0 – 75.5) (heterologous)
62.2 112 NVX half
(IQR 49.9 – 77.3) (heterologous)
72.6 Group B 106 Control
(IQR 57.6 – 77.2) (prime with
71.4 ChAd/ChAd) 107 BNT
(IQR 53.8 – 77.0) (heterologous)
71.8 109 VLA
(IQR 51.2 – 76.5) (heterologous)
71.0 111 VLA half
(IQR 51.2 – 75.9) (heterologous)
71.9 108 Ad26
(IQR 51·0 – 76.4) (heterologous)
63.5 Group B (prime 109 Control
(IQR 50·4 – 78·3) with BNT/
64·2 BNT) 110 BNT
(IQR 49·8 – 77.4) (homologous)
61.2 110 VLA
(IQR 46.2 – 77.7) (heterologous)
62.0 110 VLA half
(IQR 51.8 – 76.2) (heterologous)
61.6 106 Ad26
(IQR 49.2 – 78.3) (heterologous)
70.3 Group C (prime 114 Control
(IQR 54.4 – 75.1) with ChAd/
ChAd)
71.0 117 BNT half
(IQR 55.8 – 75.3) (heterologous)
70.2 112 m1273
(IQR 53.0 – 75.3) (heterologous)
70.3 119 CVn
(IQR 54.8 – 75.1) (heterologous)
(Cont’d...)
Volume 1 Issue 2 (2024) 20 doi: 10.36922/mi.3757