Page 30 - MI-1-2
P. 30
Microbes & Immunity Homologous versus heterologous COVID-19 vaccines
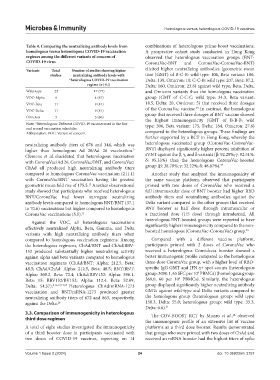
Table 4. Comparing the neutralizing antibody levels from combinations of heterologous prime-boost vaccinations.
homologous versus heterologous COVID‑19 vaccination A prospective cohort study conducted in Hong Kong
regimes among the different variants of concern of observed that heterologous vaccination groups (BNT-
COVID‑19 virus CoronaVac-BNT and CoronaVac-CoronaVac-BNT)
Variants Total Number of studies showing higher elicited higher neutralizing antibodies (geometric mean
studies neutralizing antibody levels with titer (GMT) of B-C-B: wild type: 106, Beta variant: 106,
*heterologous COVID‑19 vaccination Delta: 139, Omicron: 10; C-C-B: wild type: 207, Beta: 87.2,
regime (n [%]) Delta: 160, Omicron: 23.8) against wild type, Beta, Delta,
Wild-type 22 17 (77) and Omicron variants than the homologous vaccination
VOC-Alpha 6 4 (67) group (GMT of C-C-C: wild type: 34.3, Beta variant:
VOC-Beta 11 9 (81) 18.5, Delta: 20, Omicron: 5) that received three dosages
19
VOC-Delta 11 9 (81) of the CoronaVac vaccine. In contrast, the homologous
group that received three dosages of BNT vaccine showed
Omicron 5 3 (60)
the highest immunogenicity (GMT of B-B-B: wild
Note: *Heterologous: Different COVID-19 vaccines used in the first type: 306, Beta variant: 175, Delta: 184, Omicron: 27.6)
and second vaccination schedules.
Abbreviation: VOC: Variant of concern. compared to the heterologous groups. These findings are
further supported by a RCT in Hong Kong, whereby the
neutralizing antibody titers of 676 and 344, which was heterologous vaccinated group (CoronaVac-CoronaVac-
higher than homologous Ad 26/Ad 26 vaccination. BNT) displayed significantly higher percent inhibition of
7
Clemens et al. elucidated that heterologous vaccination sVNT against the β, γ, and δ variants (β: 92.29%; γ: 92.51%;
with CoronaVac/Ad 26, CoronaVac/BNT, and CoronaVac/ δ: 95.33%) than the homologous CoronaVac-boosted
34
ChAd all produced high neutralizing antibody titers group (β: 38.79%; γ: 32.22%; δ: 48.87%).
compared to homologous CoronaVac vaccination (211.1) Another study that analyzed the immunogenicity of
with CoronaVac/BNT vaccination having the greatest the same vaccine platform, observed that participants
geometric mean fold rise of 175.5. Another observational primed with two doses of CoronaVac who received a
12
study showed that participants who received heterologous full intramuscular dose of BNT booster had higher RBD
BNT/CoronaVac had lower surrogate neutralizing antibody titers and neutralizing antibodies against the
antibody levels compared to homologous BNT/BNT (37.1 Delta variant compared to the other groups that received
to 70.6) vaccinations but higher compared to homologous BNT booster at half dose through intramuscular or
CoronaVac vaccinations (5.5). 31 a fractional dose (1/5 dose) through intradermal. All
heterologous BNT-boosted groups were reported to have
Against the VOC, all heterologous vaccinations
effectively neutralized Alpha, Beta, Gamma, and Delta significantly higher immunogenicity compared to the non-
boosted homologous (CoronaVac-CoronaVac) group.
36
variants with high neutralizing antibody titers when
compared to homologous vaccination regimens. Among Compared with a different vaccine platform,
the heterologous regimens, ChAd/BNT and ChAd/BBV- participants primed with 2 doses of CoronaVac who
152 produced substantially higher neutralizing activity received a heterologous Convidecia booster showed a
against alpha and beta variants compared to homologous better immunogenic profile compared to the homologous
vaccination regimens (ChAd/BNT: Alpha: 212.5, Beta: three-dose CoronaVac group, with a higher level of RBD-
48.5; ChAd/ChAd: Alpha: 212.5, Beta: 48.5; BNT/BNT: specific IgG GMT and IFN-γ+ spot counts (heterologous
6
Alpha: 369.2, Beta: 72.4; ChAd/BBV152: Alpha: 396.1, group: 3090.1, 65 SFC per 10 PBMCs) (homologous group:
6
Beta: 15; BBV152/BV152: Alpha: 112.4, Beta: 52.09, 369.0, 60 per 10 PBMCs). Similarly, the heterologous
Delta: 54.37). 8,16,17,19,27 Heterologous ChAd/mRNA-1273 group displayed significantly higher neutralizing antibody
vaccination and BNT/mRNA-1273 produced greater GMTs against wild-type and Delta variants compared to
neutralizing antibody titers of 672 and 863, respectively, the homologous group (heterologous group: wild type:
against the Delta. 28 150.3, Delta: 55.0; heterologous group: wild type: 35.3,
Delta: 6.6). 21
3.3. Comparison of immunogenicity in heterologous The COV-BOOST RCT by Munro et al., observed
24
third dose regimen the immunogenic profile of an extensive list of vaccine
A total of eight studies investigated the immunogenicity platforms as a third dose booster. Results demonstrated
of a third booster dose in participants vaccinated with that groups who were primed with two doses of ChAd and
two doses of COVID-19 vaccines, reporting on 14 received an mRNA booster had the highest titers of spike
Volume 1 Issue 2 (2024) 24 doi: 10.36922/mi.3757