Page 164 - MI-2-4
P. 164
Microbes & Immunity A novel anti-EphA8 monoclonal antibody
A A
B B
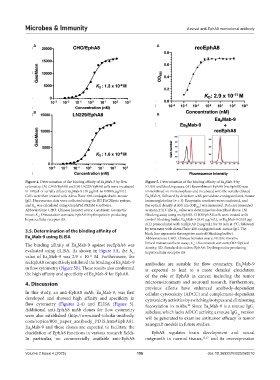
Figure 4. Determination of the binding affinity of Ea Mab-9 by flow Figure 5. Determination of the binding affinity of Ea Mab-9 by
8
8
cytometry. (A) CHO/EphA8 and (B) LN229/EphA8 cells were incubated ELISA and blocking assay. (A) Recombinant EphA8 (recEphA8) was
in 100 μL of serially diluted Ea Mab-9 (10 μg/mL to 0.0006 μg/mL). immobilized on immunoplates and incubated with the serially diluted
8
Cells were then treated with Alexa Fluor 488-conjugated anti-mouse Ea Mab-9, followed by detection with peroxidase-conjugated anti-mouse
8
IgG. Fluorescence data were collected using the BD FACSLyric system, immunoglobulins (n = 3). Enzymatic reactions were conducted, and
and K was calculated using GraphPad PRISM 6 software. the optical density at 655 nm (OD ) was measured. Data are presented
D
655
Abbreviations: CHO: Chinese hamster ovary; GeoMean: Geometric as mean ± SD. The K value was determined as described above. (B)
D
mean; K : Dissociation constant; EphA8: Erythropoietin-producing Blocking assay using recEphA8. CHO/EphA8 cells were treated with
D
hepatocellular receptor A8. control blocking buffer, Ea Mab-9 (0.01 μg/mL), or Ea Mab-9 (0.01 μg/
8
8
mL) preincubated with recEphA8 (3 μg/mL) for 30 min at 4°C, followed
3.5. Determination of the binding affinity of by treatment with Alexa Fluor 488-conjugated anti-mouse IgG. The
black line represents the negative control (blocking buffer).
Ea Mab-9 using ELISA Abbreviations: CHO: Chinese hamster ovary; ELISA: Enzyme-
8
The binding affinity of Ea Mab-9 against recEphA8 was linked immunosorbent assay; K : Dissociation constant; OD: Optical
D
density; SD: Standard deviation; EphA8: Erythropoietin-producing
8
evaluated using ELISA. As shown in Figure 5A, the K hepatocellular receptor A8.
D
value of Ea Mab-9 was 2.9 × 10 M. Furthermore, the
-11
8
recEphA8 competitively inhibited the binding of Ea Mab-9 antibodies are suitable for flow cytometry. Ea Mab-9
8
8
in flow cytometry (Figure 5B). These results also confirmed is expected to lead to a more detailed elucidation
the high affinity and specificity of Ea Mab-9 for EphA8.
8 of the role of EphA8 in cancer, including the tumor
4. Discussion microenvironment and neuronal research. Furthermore,
previous efforts have enhanced antibody-dependent
In this study, an anti-EphA8 mAb, Ea Mab-9, was first cellular cytotoxicity (ADCC) and complement-dependent
8
developed and showed high affinity and specificity in cytotoxicity activities by switching isotypes and eliminating
flow cytometry (Figures 2-4) and ELISA (Figure 5). fucosylation in mAbs. Since Ea Mab-9 is a mouse IgG
32
8
1
Additional anti-EphA8 mAb clones for flow cytometry subclass, which lacks ADCC activity, a mouse IgG version
2a
were also established (http://www.med-tohoku-antibody. will be generated to examine antitumor efficacy in tumor
com/topics/001_paper_antibody_PDIS.htm#EphA8). xenograft models in future studies.
Ea Mab-9 and these clones are expected to facilitate the
8
elucidation of EphA8 functions in various research fields. EphA8 regulates brain development and neural
In particular, no commercially available anti-EphA8 outgrowth in normal tissues, 15,17 and its overexpression
Volume 2 Issue 4 (2025) 156 doi: 10.36922/MI025060010