Page 85 - MSAM-3-1
P. 85
Materials Science in Additive Manufacturing Bioactive hydrogels for 3D bioprinting
bonds become more prevalent and shift to the right,
indicating the increase in physical interactions between
various components of the biomaterial inks. The increased
secondary bonds within the gelatin network can affect
its temperature-dependent shear thinning behavior and
viscosity. The added intermolecular physical interactions,
along with the intramolecular hydrogen bonds, affect the
conformation of the triple-helix within gelatin. Therefore,
the dissociation of the triple helix requires more energy
and the temperature of physical gelation increases. The
changes in the physical crosslinking of alginate and
physical interactions in gelatin can contribute to the further
enhancement of mechanical strength and may influence
the stability, water permeability, and swelling capacity, as
reported in our previous work and other research. 17,54 A
higher density of crosslinking can result in the rigidity of
the biomaterial inks due to the increased resistance to flow
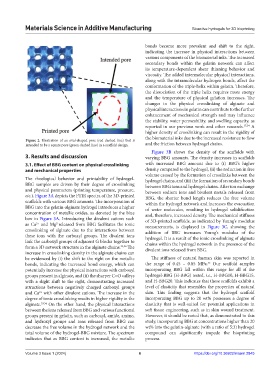
Figure 2. Illustration of an oval-shaped pore (red dashed line) that is
intended to be a square pore (green dashed line) in a scaffold design. and the friction between hydrogel chains.
Figure 3B shows the density of the scaffolds with
3. Results and discussion varying BBG amounts. The density increases in scaffolds
3.1. Effect of BBG content on physical crosslinking with increased BBG amount due to (i) BBG’s higher
and mechanical properties density compared to the hydrogel, (ii) the reduction in free
volume caused by the formation of crosslinks between the
The rheological behavior and printability of hydrogel- hydrogel chains, and (iii) the formation of secondary bonds
BBG samples are driven by their degree of crosslinking between BBG ions and hydrogel chains. After ion exchange
and physical parameters (printing temperature, pressure, between sodium ions and bivalent metals released from
etc.). Figure 3A depicts the FTIR spectra of the 3D-printed BBG, the shorter bond length reduces the free volume
scaffolds with various BBG amounts. The incorporation of within the hydrogel network and increases the evacuation
BBG into the gelatin-alginate hydrogel introduces a higher of water molecules, resulting in hydrogel solidification
concentration of metallic oxides, as denoted by the blue and, therefore, increased density. The mechanical stiffness
box in Figure 3A. Introducing the divalent cations such of 3D-printed scaffolds, as indicated by Young’s modulus
as Ca and Mg released from BBG facilitates the ionic measurements, is displayed in Figure 3C, showing the
2+
2
crosslinking of alginate due to the interactions between addition of BBG increases Young’s modulus of the
these ions with the carboxyl groups. The divalent ions hydrogel. It is a result of the ionic crosslinking of alginate
link the carboxyl groups of adjacent G blocks together to chains within the hydrogel network in the presence of the
form a 3D network structure in the alginate chains. 34,36 The divalent ions released from BBG.
increase in crosslinking density in the alginate chains can
be evidenced by (i) the shift to the right on the metallic The stiffness of natural human skin was reported in
bonds, indicating the increased bond energy, which can the range of 0.45 – 0.85 MPa. Our scaffold samples
55
potentially increase the physical interactions with carboxyl incorporating BBG fall within this range for all of the
groups present in alginate, and (ii) the sharper C=O valleys hydrogel-BBG (H-BBG) tested, i.e., H-BBG10, H-BBG15,
with a slight shift to the right, demonstrating increased and H-BBG20. This indicates that these scaffolds exhibit a
attractions between negatively charged carboxyl groups level of elasticity that resembles the properties of natural
and Ca with other divalent cations. The increase in the skin. This finding suggests that the hydrogel scaffold
2+
degree of ionic crosslinking results in higher rigidity in the incorporating BBG up to 20 wt% possesses a degree of
alginate. 53,54 On the other hand, the physical interactions elasticity that is well-suited for potential applications in
between the ions released from BBG and various functional soft tissue engineering, such as in skin wound treatment.
groups present in gelatin, such as carboxyl, amide, amino, However, it should be noted that, as demonstrated in this
and hydroxyl groups and ions released from BBG can study, incorporating BBG at concentrations higher than 20
decrease the free volume in the hydrogel network and the wt% into the gelatin-alginate (with a ratio of 5:3) hydrogel
total volume of the hydrogel-BBG mixture. The spectrum compound can significantly impede the bioprinting
indicates that as BBG content is increased, the metallic process.
Volume 3 Issue 1 (2024) 6 https://doi.org/10.36922/msam.2845