Page 86 - MSAM-3-1
P. 86
Materials Science in Additive Manufacturing Bioactive hydrogels for 3D bioprinting
A
B
C
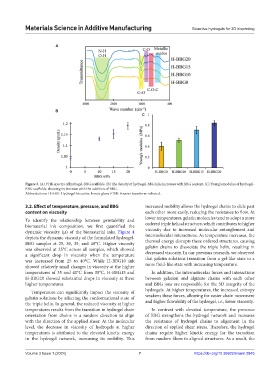
Figure 3. (A) FTIR spectra of hydrogel-BBG scaffolds. (B) The density of hydrogel-BBG inks increases with BBG content. (C) Young’s modulus of hydrogel-
BBG scaffolds, showing its increase with the addition of BBG.
Abbreviations: H-BBG: Hydrogel-bioactive borate glass; FTIR: Fourier transform infrared.
3.2. Effect of temperature, pressure, and BBG increased mobility allows the hydrogel chains to slide past
content on viscosity each other more easily, reducing the resistance to flow. At
lower temperatures, gelatin molecules tend to adopt a more
To identify the relationship between printability and
biomaterial ink composition, we first quantified the ordered triple helical structure, which contributes to higher
dynamic viscosity (µ) of the biomaterial inks. Figure 4 viscosity due to increased molecular entanglement and
depicts the dynamic viscosity of the formulated hydrogel- intermolecular interactions. As temperature increases, the
BBG samples at 25, 30, 35, and 40°C. Higher viscosity thermal energy disrupts these ordered structures, causing
was observed at 25°C across all samples, which showed gelatin chains to dissociate the triple helix, resulting in
a significant drop in viscosity when the temperature decreased viscosity. In our previous research, we observed
was increased from 25 to 40°C. While H-BBG10 ink that gelatin solutions transition from a gel-like state to a
showed relatively small changes in viscosity at the higher more fluid-like state with increasing temperature.
temperatures of 35 and 40°C from 30°C, H-BBG15 and In addition, the intermolecular forces and interactions
H-BBG20 showed substantial drops in viscosity at these between gelation and alginate chains with each other
higher temperatures. and BBG ions are responsible for the 3D integrity of the
Temperature can significantly impact the viscosity of hydrogels. At higher temperatures, the increased entropy
gelatin solutions by affecting the conformational state of weakens these forces, allowing for easier chain movement
the triple helix. In general, the reduced viscosity at higher and higher flowability of the hydrogel, i.e., lower viscosity.
temperatures results from the transition in hydrogel chain In contrast with elevated temperature, the presence
orientation from chains in a random direction to align of BBG strengthens the hydrogel network and increases
with the direction of the applied shear. At the molecular the resistance of hydrogel chains to alignment in the
level, the decrease in viscosity of hydrogels at higher direction of applied shear stress. Therefore, the hydrogel
temperatures is attributed to the elevated kinetic energy chains require higher kinetic energy for the transition
in the hydrogel network, increasing its mobility. This from random fibers to aligned structures. As a result, the
Volume 3 Issue 1 (2024) 7 https://doi.org/10.36922/msam.2845