Page 99 - MSAM-4-2
P. 99
Materials Science in Additive Manufacturing Additively manufactured high carbon steel
A B
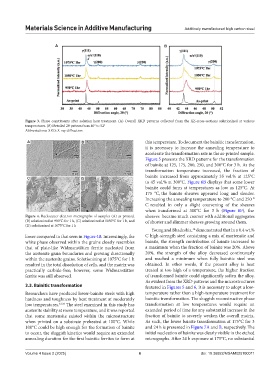
Figure 3. Phase constituents after solution heat treatment. (A) Overall XRD patterns collected from the XZ-cross-sections solutionized at various
temperatures. (B) Detailed 2θ pattern from 40° to 52°
Abbreviations: XRD; X-ray diffraction
A B this temperature. To document the bainitic transformation,
it is necessary to increase the annealing temperature to
accelerate the transformation rate in the as-printed sample.
Figure 5 presents the XRD patterns for the transformation
of bainite at 125, 175, 200, 250, and 300°C for 3 h. As the
transformation temperature increased, the fraction of
C D bainite increased from approximately 35 vol.% at 125°C
to 45 vol.% at 300°C. Figure 6B displays that some lower
bainite could form at temperatures as low as 125°C. At
175 °C, the bainite sheaves appeared long and slender.
Increasing the annealing temperature to 200 °C and 250 °
C resulted in only a slight coarsening of the sheaves
when transformed at 300°C for 3 h (Figure 6F), the
Figure 4. Backscatter electron micrographs of samples (A) as printed, sheaves became much coarser with additional aggregates
(B) solutionized at 950°C for 1 h, (C) solutionized at 1050°C for 1 h, and of shorter and slimmer sheaves growing around them.
(D) solutionized at 1075°C for 1 h
Young and Bhadeshia. demonstrated that in a 0.4 wt.%
34
lower compared to that seen in Figure 4B. Interestingly, the C high-strength steel containing a mix of martensite and
white phase observed within the grains closely resembles bainite, the strength contribution of bainite increased to
that of plate-like Widmanstätten ferrite nucleated from a maximum when the fraction of bainite was 20%. Above
the austenite grain boundaries and growing directionally 20%, the strength of the alloy decreased continuously
within the austenite grains. Solutionizing at 1075°C for 1 h and reached a minimum when fully bainitic steel was
resulted in the total dissolution of cells, and the matrix was obtained. In other words, if the present alloy is heat
practically carbide-free; however, some Widmanstätten treated at too high of a temperature, the higher fraction
ferrite was still observed. of transformed bainite could significantly soften the alloy.
As evident from the XRD patterns and the microstructures
3.3. Bainitic transformation featured in Figures 5 and 6, it is necessary to adopt a low-
Researchers have produced lower-bainite steels with high temperature rather than a high-temperature treatment for
hardness and toughness by heat treatment at moderately bainitic transformation. The sluggish reconstructive phase
low temperatures. 32,33 The steel examined in this study has transformation at low temperatures would require an
austenite stability at room temperature, and it was reported extended period of time for any substantial increase in the
that some martensite existed within the microstructure fraction of bainite to severely weaken the overall matrix.
when printed on a substrate preheated at 100°C. While As such, the lower bainite transformation at 175°C for 3
100°C could be high enough for the formation of bainite and 24 h is presented in Figure 7A and B, respectively. The
to occur, the sluggish kinetics would require an extended initial nucleation of bainite was clearly visible in the etched
annealing duration for the first bainitic ferrites to form at micrographs. After 24 h exposure at 175°C, no substantial
Volume 4 Issue 2 (2025) 6 doi: 10.36922/MSAM025100011