Page 30 - OR-1-2
P. 30
70 – 85% of its volume but only 25 – 35% of its total cells. were encapsulated within a microfibrous structure created
88
The remaining 65 – 75% consists of non-cardiomyocytes, by bioprinting, leading to a continuous endothelial layer
including endothelial cells, vascular smooth muscle cells, (Figure 2B). The hydrogel scaffold facilitated the orderly
fibroblasts, neurons, and immune cells. These heart cells localization of endothelial cells within the microfibrous
89
and the vascular system are surrounded by a 3D ECM structure. Subsequently, myocardial cells were seeded
network. The ECM is composed of collagen, laminin, and onto the macroscale anisotropic microfibrous structures,
fibronectin secreted by heart cells, along with a plethora inducing the formation of well-aligned myocardial tissue
of cell adhesion molecules, growth factors, proteases, and capable of spontaneous and synchronized contraction
glycoproteins. 90,91 Organoid hydrogels are ideal for cardiac (Figure 2C). Coupled with a microfluidic perfusion
scaffolds due to their customizability, unique mechanical bioreactor, the endothelialized myocardial chip was used
properties, controlled release of biological factors, and to screen for the cardiovascular toxicity of drugs. Finally,
the ability to replicate the heart’s structure, function, and the potential of translating this model into endothelialized
complex microenvironment. human myocardial tissue was explored, using hiPSC-
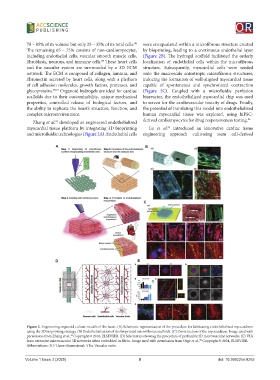
Zhang et al. developed an engineered endothelialized derived cardiomyocytes for drug responsiveness testing. 92
92
myocardial tissue platform by integrating 3D bioprinting Lu et al. introduced an innovative cardiac tissue
93
and microfluidic technologies (Figure 2A). Endothelial cells engineering approach cultivating stem cell-derived
A B
C
D E
Figure 2. Engineering organoid culture models of the heart. (A) Schematic representation of the procedure for fabricating endothelialized myocardium
using the 3D bioprinting strategy. (B) Endothelialization of the bioprinted microfibrous scaffolds. (C) Construction of the myocardium. Image used with
permission from Zhang et al., Copyright © 2016, ELSEVIER. (D) Schematics showing the procedure of perfusable 3D microvascular networks. (E) VUs
92
form extensive microvascular 3D networks when embedded in fibrin. Image used with permission from Orge et al., Copyright © 2024, ELSEVIER.
98
Abbreviations: 3D: Three-dimensional; VUs: Vascular units.
Volume 1 Issue 2 (2025) 9 doi: 10.36922/or.8262