Page 22 - AJWEP-22-5
P. 22
Rajak, et al.
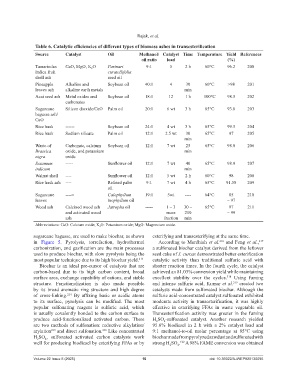
Table 6. Catalytic efficiencies of different types of biomass ashes in transesterification
Source Catalyst Oil Methanol/ Catalyst Time Temperature Yield References
oil ratio load (%)
Tamarindus CaO, MgO, K O Parinari 9:1 5 2 h 60°C 96.2 200
2
indica fruit curatellifolia
shell ash seed oil
Pineapple Alkaline and Soybean oil 40:1 4 30 60°C >98 201
leaves ash alkaline earth metals min
Acai seed ash Metal oxides and Soybean oil 18:1 12 1 h 100°C 98.5 202
carbonates
Sugarcane Silicon dioxide/CaO Palm oil 20:1 6 wt 3 h 65°C 93.8 203
bagasse ash/
CaO
Rice husk ------ Soybean oil 24:1 4 wt 3 h 65°C 99.5 204
Rice husk Sodium silicate Palm oil 12:1 2.5 wt 30 65°C 97 205
min
Waste of Carbonate, calcium Soybean oil 12:1 7 wt 25 65°C 98.8 206
Brassica oxide, and potassium min
nigra oxide
Sesamum ----- Sunflower oil 12:1 7 wt 40 65°C 98.9 207
indicum min
Walnut shell ---- Sunflower oil 12:1 5 wt 2 h 60°C 98 208
Rice husk ash ---- Refined palm 9:1 7 wt 4 h 65°C 91.58 209
oil
Sugarcane ----= Calophyllum 19:1 5wt ---- 64°C 85 210
leaves inophyllum oil – 97
Wood ash Calcined wood ash Jatropha oil ----- 1 – 3 30 – 65°C 97 211
and activated wood mass 210 – 99
ash fraction min
Abbreviations: CaO: Calcium oxide; K 2 O: Potassium oxide; MgO: Magnesium oxide.
sugarcane bagasse, are used to make biochar, as shown esterifying and transesterifying at the same time.
in Figure 5. Pyrolysis, torrefaction, hydrothermal According to Mardhiah et al. and Feng et al.,
216
217
carbonization, and gasification are the main processes a sulfonated biochar catalyst derived from the leftover
used to produce biochar, with slow pyrolysis being the seed cake of J. curcas demonstrated better esterification
most popular technique due to its high biochar yield. 213 catalytic activity than traditional sulfuric acid with
Biochar is an ideal pre-cursor of catalysts that are shorter reaction times. In the fourth cycle, the catalyst
carbon-based due to its high carbon content, broad achieved an 81.03% conversion yield while maintaining
surface area, exchange capability of cations, and stable excellent stability over the cycles. Using fuming
218
structure. Functionalization is also made possible and intense sulfuric acid, Kumar et al. created two
219
by its broad aromatic ring structure and high degree catalysts made from sulfonated biochar. Although the
of cross-linking. By affixing basic or acidic atoms sulfuric acid-concentrated catalyst sulfonated exhibited
214
to its surface, pyrolysis can be modified. The most moderate activity in transesterification, it was highly
popular sulfonating reagent is sulfuric acid, which effective in esterifying FFAs in waste vegetable oil.
is usually covalently bonded to the carbon surface to Transesterification activity was greater in the fuming
produce acid-functionalized activated carbon. There H SO -sulfonated catalyst. Another research yielded
2
4
are two methods of sulfonation: reductive alkylation/ 95.6% biodiesel in 2 h with a 2% catalyst load and
arylation and direct sulfonation. Like concentrated 9:1 methanol-to-oil molar percentage at 85°C using
215
216
H SO , sulfonated activated carbon catalysts work biochar made from pyrolyzed sawdust and sulfonated with
2
4
well for producing biodiesel by esterifying FFAs or by strong H SO . A 98% FAME conversion was obtained
122
2
4
Volume 22 Issue 5 (2025) 16 doi: 10.36922/AJWEP025130095