Page 79 - AN-3-4
P. 79
Advanced Neurology SARS-CoV-2 in age-associated neurodegeneration
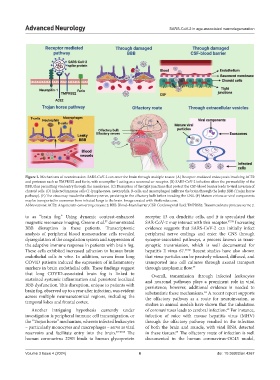
Figure 2. Mechanisms of neuroinvasion. SARS-CoV-2 can enter the brain through multiple routes: (A) Receptor-mediated endocytosis involving ACE2
and proteases such as TMPRSS2 and furin, with neuropilin-1 acting as a neuronal co-receptor. (B) SARS-CoV-2 infection alters the permeability of the
BBB, thus permitting viral entry through the membrane. (C) Disruption of the tight junctions that protect the CSF-blood barrier leads to viral invasion of
choroid cells. (D) Infected immune cells (T-lymphocytes, neutrophils, B-cells, and macrophages) infiltrate the brain through the leaky BBB (Trojan horse
pathway). (E) The virus may invade the olfactory nerve, persisting in the olfactory bulb before invading the CNS. (F) Mature virions or viral components
may be transported in exosomes from infected lungs to the brain. Image created with BioRender.com.
Abbreviations: ACE2: Angiotensin-converting enzyme 2; BBB: Blood–brain barrier; CSF: Cerebrospinal fluid; TMPRSS2: Transmembrane protease serine 2.
to as “brain fog.” Using dynamic contrast-enhanced receptor 13 on dendritic cells, and it is speculated that
magnetic resonance imaging, Greene et al. demonstrated SAR-CoV-2 may interact with this receptor. 47,59 Increasing
57
BBB disruption in these patients. Transcriptomic evidence suggests that SARS-CoV-2 can initially infect
analysis of peripheral blood mononuclear cells revealed peripheral nerve endings and enter the CNS through
dysregulation of the coagulation system and suppression of synapse-associated pathways, a process known as trans-
the adaptive immune response in patients with brain fog. synaptic transmission, which is well documented for
These cells exhibited increased adhesion to human brain hepatitis E virus 67. 47,60 Recent studies have also shown
endothelial cells in vitro. In addition, serum from long that virus particles can be passively released, diffused, and
COVID patients induced the expression of inflammatory transported into cell cultures through axonal transport
markers in brain endothelial cells. These findings suggest through axoplasmic flow. 47
that long COVID-associated brain fog is linked to Overall, transmission through infected leukocytes
sustained systemic inflammation and persistent localized and neuronal pathways plays a prominent role in viral
BBB dysfunction. This disruption, unique to patients with persistence; however, additional evidence is needed to
brain fog, observed up to a year after infection, was evident substantiate these mechanisms. A recent report supports
61
across multiple neuroanatomical regions, including the the olfactory pathway as a route for neuroinvasion, as
temporal lobes and frontal cortex. studies in animal models have shown that the inhalation
Another intriguing hypothesis currently under of coronaviruses leads to cerebral infection. For instance,
62
investigation is peripheral immune cell transmigration, or infection of mice with mouse hepatitis virus (MHV)
the “Trojan horse” mechanism, wherein infected leukocytes through the olfactory pathway resulted in the infection
– particularly monocytes and macrophages – serve as viral of both the brain and muscle, with viral RNA detected
reservoirs and facilitate entry into the brain. 47,48,58 The in these tissues. The olfactory route of infection is well
63
human coronavirus 229E binds to human glycoprotein documented in the human coronavirus-OC43 model,
Volume 3 Issue 4 (2024) 6 doi: 10.36922/an.4267