Page 59 - EER-2-3
P. 59
Explora: Environment
and Resource Electrocatalyst for ammonia oxidation reaction
A B C
D
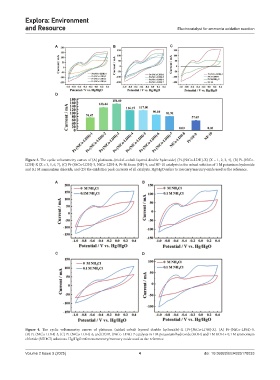
Figure 3. The cyclic voltammetry curves of (A) platinum–(nickel–cobalt layered double hydroxide) (Pt-[NiCo-LDH]-X) (X = 1, 2, 3, 4), (B) Pt-(NiCo-
LDH)-X (X = 3, 5, 6, 7), (C) Pt-(NiCo-LDH)-3, NiCo-LDH-8, Pt-Ni foam (NF)-9, and NF-10 catalysts in the mixed solution of 1 M potassium hydroxide
and 0.1 M ammonium chloride, and (D) the oxidation peak currents of all catalysts. Hg/HgO refers to mercury/mercury oxide used as the reference.
A B
C D
Figure 4. The cyclic voltammetry curves of platinum–(nickel–cobalt layered double hydroxide)-X (Pt-[NiCo-LDH]-X). (A) Pt-(NiCo-LDH)-3,
(B) Pt-(NiCo-LDH)-5, (C) Pt-(NiCo-LDH)-6, and (D) Pt-(NiCo-LDH)-7 catalysts in 1 M potassium hydroxide (KOH) and 1 M KOH + 0.1 M ammonium
chloride (NH4Cl) solutions. Hg/HgO refers to mercury/mercury oxide used as the reference.
Volume 2 Issue 3 (2025) 4 doi: 10.36922/EER025170033