Page 62 - EER-2-3
P. 62
Explora: Environment
and Resource Electrocatalyst for ammonia oxidation reaction
Table 1. The ammonia oxidation reaction test conditions and activity of reported catalysts
Electrode Onset potential (V) Current density (mA/cm ) Electrolyte Stability References
2
Platinum/cobalt–iron/nickel −0.019 V RHE 50.00 at 0.029 V RHE 0.2 M N H 1.0 M KOH At −0.017 V RHE for 23
+
2
4
foam 100 h
Platinum–iridium–copper 0.350 V 40.60 at 0.500 V 0.1 M NH 1.0 M KOH At 0.650 V for 24
+
RHE RHE 3 RHE
500 s
Silver/iron (II) oxide/titanate −0.500 V Ag/AgCl - 0.1 M NH4Cl 1.0 M At −0.250 V Ag/AgCl for 25
+
nanotubes phosphate-buffered saline five cycles
+
Platinum–cobalt hydroxide– 0.250 V RHE 10.17 at − 0.200 V RHE 0.1 M NH4Cl 1.0 M KOH - 31
nickel foam-3
+
Nickel–copper–sulfur treated/ 1.374 V RHE 110.00 at 1.690 V RHE 0.2 M NH4Cl 1.0 M NaOH At 1.640 V RHE for 27
carbon paper 24 h
Platinum–iridium 0.350 V RHE 32.20 at 0.500 V RHE 1.0 M ammonia 1.0 M KOH At 0.500–1.000 V RHE 28
+
(5:5 atomic ratio)/XC-72 for 4,000 cycles
Platinum–(nickel–cobalt −0.03 V Hg/HgO 154.60 at 0.24 V Hg/HgO 0.1 M NH Cl 1.0 M KOH At 0.24 VHg/HgO for This work
+
4
layered double hydroxide)-3 5,000 s
Abbreviations: Ag: Silver; AgCl: Silver chloride; Hg: Mercury; HgO: Mercury oxide; KOH: Potassium chloride; NH Cl: Ammonium chloride;
4
RHE: Reversible hydrogen electrode.
A B
C D
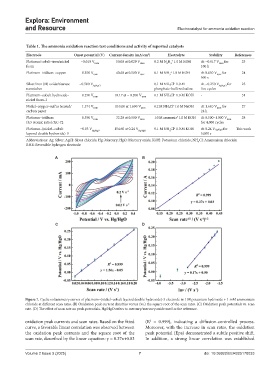
Figure 7. Cyclic voltammetry curves of platinum–(nickel–cobalt layered double hydroxide)-3 electrode in 1 M potassium hydroxide + 1 mM ammonium
chloride at different scan rates. (B) Oxidation peak current densities versus (vs.) the square root of the scan rates. (C) Oxidation peak potentials vs. scan
rate. (D) The effect of scan rate on peak potentials. Hg/HgO refers to mercury/mercury oxide used as the reference.
oxidation peak currents and scan rates. Based on the fitted (R = 0.999), indicating a diffusion-controlled process.
2
curve, a favorable linear correlation was observed between Moreover, with the increase in scan rates, the oxidation
the oxidation peak currents and the square root of the peak potential (Epa) demonstrated a subtle positive shift.
scan rate, described by the linear equation: y = 0.37x+0.03 In addition, a strong linear correlation was established
Volume 2 Issue 3 (2025) 7 doi: 10.36922/EER025170033