Page 60 - EER-2-3
P. 60
Explora: Environment
and Resource Electrocatalyst for ammonia oxidation reaction
approximately −0.05 V. Figure 3A shows the CV curves of oxidation peak current gradually decreased. The oxidation
Pt-(NiCo-LDH)-1, Pt-(NiCo-LDH)-2, Pt-(NiCo-LDH)-3, peak currents decreased from 154.60 mA to 81.91 mA
and Pt-(NiCo-LDH)-4 catalysts in the mixed solution of 1 (Figure 3D), indicating that the catalytic activity gradually
M KOH and 0.1 M NH Cl, with oxidation peak currents weakened. Increasing reaction temperatures accelerated
4
shown in Figure 3D. Notably, with an increasing volume the rate of the displacement reaction between PtCl and
2-
6
of H PtCl 6H O, the oxidation peak current exhibited Co . Simultaneously, the nucleation rate of Pt was also
2+
2
6
2
a notable upward trend, escalating from 74.47 mA to accelerated, which hindered the formation of highly
154.60 mA, indicating an enhanced catalytic activity. dispersed Pt and reduced the number of catalytically active
However, at 0.6 mL, the catalytic activity of Pt-(NiCo- sites.
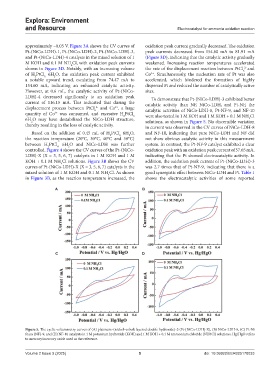
LDH)-4 decreased significantly to an oxidation peak To demonstrate that Pt-(NiCo-LDH)-3 exhibited better
current of 116.15 mA. This indicated that during the catalytic activity than NF, NiCo-LDH, and Pt-NF, the
displacement process between PtCl and Co , a large catalytic activities of NiCo-LDH-8, Pt-NF-9, and NF-10
2-
2+
6
quantity of Co was consumed, and excessive H PtCl were also tested in 1 M KOH and 1 M KOH + 0.1 M NH Cl
2+
2
6
6H O may have destabilized the NiCo-LDH structure, solutions, as shown in Figure 5. No discernible variation
4
2
thereby resulting in the loss of catalytic activity. in current was observed in the CV curves of NiCo-LDH-8
Based on the addition of 0.45 mL of H PtCl 6H O, and NF-10, indicating that pure NiCo-LDH and NF did
6
2
2
the reaction temperature (20°C, 30°C, 40°C and 50°C) not show obvious catalytic activity in this measurement
between H PtCl 6H O and NiCo-LDH was further system. In contrast, the Pt-NF-9 catalyst exhibited a clear
2
6
2
controlled. Figure 4 shows the CV curves of the Pt-(NiCo- oxidation peak with an oxidation peak current of 57.65 mA,
LDH)-X (X = 3, 5, 6, 7) catalysts in 1 M KOH and 1 M indicating that the Pt showed electrocatalytic activity. In
KOH + 0.1 M NH Cl solutions. Figure 3B shows the CV addition, the oxidation peak current of Pt-(NiCo-LDH)-3
4
curves of Pt-(NiCo-LDH)-X (X = 3, 5, 6, 7) catalysts in the was 2.7 times that of Pt-NF-9, indicating that there is a
mixed solution of 1 M KOH and 0.1 M NH Cl. As shown good synergistic effect between NiCo-LDH and Pt. Table 1
4
in Figure 3B, as the reaction temperature increased, the shows the electrocatalytic activities of some reported
A B
C D
Figure 5. The cyclic voltammetry curves of (A) platinum–(nickel–cobalt layered double hydroxide)-3 (Pt-[NiCo-LDH]-X), (B) NiCo-LDH-8, (C) Pt-Ni
foam (NF)-9, and (D) NF-10 catalysts in 1 M potassium hydroxide (KOH) and 1 M KOH + 0.1 M ammonium chloride (NH4Cl) solutions. Hg/HgO refers
to mercury/mercury oxide used as the reference.
Volume 2 Issue 3 (2025) 5 doi: 10.36922/EER025170033