Page 64 - EER-2-3
P. 64
Explora: Environment
and Resource Electrocatalyst for ammonia oxidation reaction
Three analyses, SEM, X-ray diffraction (XRD, D8 LDH)-3 showed more apparent changes, indicating that
advance, Bruker, Germany), and XPS, were employed the NiCo-LDH underwent phase transitions and lattice
to study the morphology and composition of Pt-(NiCo- rearrangements during the electrochemical measurement
LDH)-3. Figure 9A and B show the SEM images of process.
NiCo-LDH. It was observed that NiCo-LDH existed Figure 10 exhibits the XRD pattern of Pt-(NiCo-
in the form of nanowires, with a certain degree of LDH)-3. As shown in Figure 7, the three strong diffraction
agglomeration. At a lower magnification (Figure 9B), peaks at 44.7°, 52.0°, and 76.5° could be attributed to NF.
it was easy to observe that these nanowires combined In addition, three diffraction peaks could be detected at
to form a flower-like morphology. However, after the 40.5°, 47.1°, and 68.7°, attributable to the 111, 200, and 220
growth of Pt after incubation in the water bath, the crystal planes of Pt. In addition, a weaker diffraction peak
30
flower-like structure formed by the agglomeration of of NiCo-LDH was observed at 22.8°. The XRD diffraction
24
these NiCo-LDH nanowires degraded, as shown in peaks of NiCo-LDH and Pt confirm the successful growth
Figure 9C and D. This could be attributed to two aspects:
first, the addition of H PtCl 6H O introduces a large of NiCo-LDH and Pt on the surface of NF.
2
6
2
number of H , which provides a certain corrosive effect To further characterize the chemical composition
+
on NiCo-LDH, and second, the Co ions within NiCo- of Pt-(NiCo-LDH)-3, XPS was performed, and the
3+
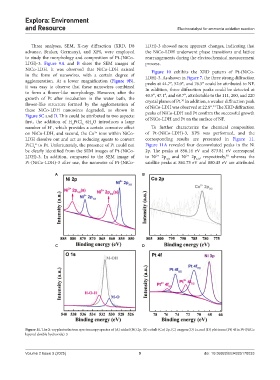
LDH dissolve out and act as reducing agents to convert corresponding results are presented in Figure 11.
2-
PtCl to Pt. Unfortunately, the presence of Pt could not Figure 11A revealed four deconvoluted peaks in the Ni
6
be clearly identified from the SEM images of Pt-(NiCo- 2p. The peaks at 856.14 eV and 873.81 eV correspond
LDH)-3. In addition, compared to the SEM image of to Ni 2p and Ni 2p , respectively, whereas the
2+
2+
30
3/2
1/2
Pt-(NiCo-LDH)-3 after use, the nanowire of Pt-(NiCo- satellite peaks at 861.73 eV and 880.43 eV are attributed
A B
C D
Figure 11. The X-ray photoelectron spectroscopy spectra of (A) nickel (Ni) 2p, (B) cobalt (Co) 2p, (C) oxygen (O) 1s, and (D) platinum (Pt) 4f in Pt-(NiCo
layered double hydroxide)-3
Volume 2 Issue 3 (2025) 9 doi: 10.36922/EER025170033