Page 61 - EER-2-3
P. 61
Explora: Environment
and Resource Electrocatalyst for ammonia oxidation reaction
catalysts. Compared to these catalysts, the onset potential the Pt catalyst, revealing the potential range more suitable
and current density of Pt-(NiCo-LDH)-3 were accepted. for NiCo-LDH. However, as shown in Figure 4C and D, the
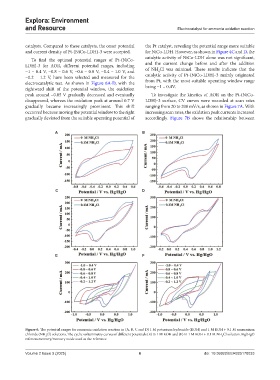
To find the optimal potential ranges of Pt-(NiCo- catalytic activity of NiCo-LDH alone was not significant,
LDH)-3 for AOR, different potential ranges, including and the current change before and after the addition
−1 – 0.4 V, −0.8 – 0.6 V, −0.6 – 0.8 V, −0.4 – 1.0 V, and of NH Cl was minimal. These results indicate that the
4
−0.2 – 1.2 V, have been selected and measured for the catalytic activity of Pt-(NiCo-LDH)-3 mainly originated
electrocatalytic test. As shown in Figure 6A-D, with the from Pt, with the most suitable operating window range
rightward shift of the potential window, the oxidation being −1 – 0.4V.
peak around −0.05 V gradually decreased and eventually To investigate the kinetics of AOR on the Pt-(NiCo-
disappeared, whereas the oxidation peak at around 0.7 V LDH)-3 surface, CV curves were recorded at scan rates
gradually became increasingly prominent. This shift ranging from 20 to 200 mV/s, as shown in Figure 7A. With
occurred because moving the potential window to the right increasing scan rates, the oxidation peak currents increased
gradually deviated from the suitable operating potential of accordingly. Figure 7B shows the relationship between
A B
C D
E F
Figure 6. The potential ranges for ammonia oxidation reaction in (A, B, C and D) 1 M potassium hydroxide (KOH) and 1 M KOH + 0.1 M ammonium
chloride (NH Cl) solutions. The cyclic voltammetry curves of different potentials (E) in 1 M KOH and (F) in 1 M KOH + 0.1 M NH Cl solution. Hg/HgO
4
4
refers to mercury/mercury oxide used as the reference.
Volume 2 Issue 3 (2025) 6 doi: 10.36922/EER025170033