Page 122 - EJMO-9-3
P. 122
Eurasian Journal of
Medicine and Oncology Zercepac + pyrotinib versus pertuzumab in HER2+ BC
®
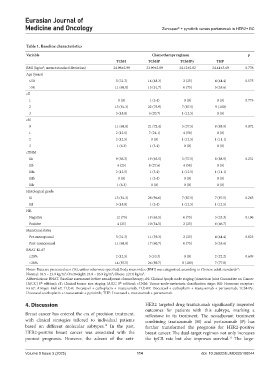
Table 1. Baseline characteristics
Variable Chemotherapy regimen p
TCbH TCbHP TCbHPy THP
BMI (kg/m ; mean±standard deviation) 24.96±2.99 23.99±2.89 24.12±2.82 24.41±3.69 0.776
2
Age (years)
≤50 5 (31.3) 14 (48.3) 2 (25) 4 (44.4) 0.575
>50 11 (68.8) 15 (51.7) 6 (75) 5 (55.6)
cT
1 0 (0) 1 (3.4) 0 (0) 0 (0) 0.773
2 13 (81.3) 22 (75.9) 7 (87.5) 9 (100)
3 3 (18.8) 6 (20.7) 1 (12.5) 0 (0)
cN
0 11 (68.8) 21 (72.4) 3 (37.5) 8 (88.9) 0.072
1 2 (12.5) 7 (24.1) 4 (50) 0 (0)
2 2 (12.5) 0 (0) 1 (12.5) 1 (11.1)
3 1 (6.3) 1 (3.4) 0 (0) 0 (0)
cTNM
IIa 9 (56.3) 19 (65.5) 3 (37.5) 8 (88.9) 0.231
IIb 4 (25) 8 (27.6) 4 (50) 0 (0)
IIIa 2 (12.5) 1 (3.4) 1 (12.5) 1 (11.1)
IIIb 0 (0) 1 (3.4) 0 (0) 0 (0)
IIIc 1 (6.3) 0 (0) 0 (0) 0 (0)
Histological grade
II 13 (81.3) 28 (96.6) 7 (87.5) 7 (87.5) 0.265
III 3 (18.8) 1 (3.4) 1 (12.5) 1 (12.5)
HR
Negative 12 (75) 19 (65.5) 6 (75) 3 (33.3) 0.196
Positive 4 (25) 10 (34.5) 2 (25) 6 (66.7)
Menstrual status
Pre-menopausal 5 (31.3) 11 (39.3) 2 (25) 4 (44.4) 0.823
Post-menopausal 11 (68.8) 17 (60.7) 6 (75) 5 (55.6)
BNAT Ki-67
≤20% 2 (12.5) 3 (10.3) 0 (0) 2 (22.2) 0.609
>20% 14 (87.5) 26 (89.7) 8 (100) 7 (77.8)
Notes: Data are presented as n (%), unless otherwise specified; Body mass index (BMI) was categorized according to Chinese adult standards :
17
Normal: 18.5 – 23.9 kg/m ; Overweight: 24.0 – 26.9 kg/m ; Obese: ≥27.0 kg/m . 2
2
2
Abbreviations: BNAT: Baseline assessment before neoadjuvant chemotherapy; cN: Clinical lymph node staging (American Joint Committee on Cancer
[AJCC] 8 edition); cT: Clinical tumor size staging (AJCC 8 edition); cTNM: Tumor-node-metastasis classification stage; HR: Hormone receptor;
th
th
Ki-67: Antigen Kiel 67; TCbH: Docetaxel + carboplatin + trastuzumab; TCbHP: Docetaxel + carboplatin + trastuzumab + pertuzumab; TCbHPy:
Docetaxel+carboplatin + trastuzumab + pyrotinib; THP: Docetaxel + trastuzumab + pertuzumab.
4. Discussion HER2 targeted drug trastuzumab significantly improved
outcomes for patients with this subtype, marking a
Breast cancer has entered the era of precision treatment, milestone in its treatment. The neoadjuvant treatment
with clinical strategies tailored to individual patients combining trastuzumab (H) and pertuzumab (P) has
10
based on different molecular subtypes. In the past, further transformed the prognosis for HER2-positive
HER2-positive breast cancer was associated with the breast cancer. The dual-target regimen not only increases
poorest prognosis. However, the advent of the anti- the tpCR rate but also improves survival. The large-
11
Volume 9 Issue 3 (2025) 114 doi: 10.36922/EJMO025100044