Page 123 - EJMO-9-3
P. 123
Eurasian Journal of
Medicine and Oncology Zercepac + pyrotinib versus pertuzumab in HER2+ BC
®
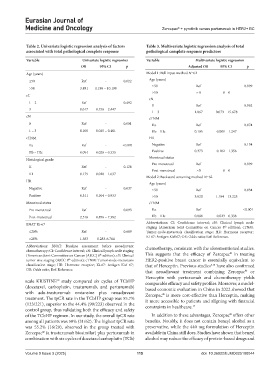
Table 2. Univariate logistic regression analysis of factors Table 3. Multivariate logistic regression analysis of total
associated with total pathological complete response pathological complete response predictors
Variable Univariate logistic regression Variable Multivariate logistic regression
OR 95% CI p Adjusted OR 95% CI p
Age (years) Model 1: Full input method N=62
≤50 Ref - 0.022 Age (years)
≤50 Ref - 0.999
>50 3.491 1.196 – 10.190
>50 ≈ 0 0 – 0
cT
cN
1 – 2 Ref - 0.492
0 Ref - 0.962
3 0.617 0.156 – 2.447
1 – 3 1.067 0.073 – 15.678
cN cTNM
0 Ref - 0.001 IIa Ref - 0.074
1 – 3 0.100 0.025 – 0.401 IIb – IIIc 0.105 0.009 – 1.247
cTNM HR
IIa Ref - <0.001 Negative Ref - 0.134
IIb – IIIc 0.094 0.026 – 0.335 Positive 0.373 0.102 – 1.356
Menstrual status
Histological grade
Pre-menstrual Ref - 0.999
II Ref - 0.128
Post-menstrual ≈0 0 – 0
III 0.179 0.020 – 1.637
Model 2: Backward screening method N=62
HR Age (years)
Negative Ref - 0.037 ≤50 Ref - 0.034
Positive 0.311 0.104 – 0.933 >50 3.820 1.104 – 13.225
Menstrual status cTNM
Pre-menstrual Ref - 0.093 IIa Ref - <0.001
Post-menstrual 2.516 0.856 – 7.392 IIb – IIIc 0.088 0.023 – 0.338
BNAT Ki-67 Abbreviations: CI: Confidence interval; cN: Clinical lymph node
th
staging (American Joint Committee on Cancer 8 edition); cTNM:
≤20% Ref - 0.689 Tumor-node-metastasis classification stage; HR: Hormone receptor;
>20% 1.383 0.283-6.764 Ki-67: Antigen Kiel 67; OR: Odds ratio; Ref: Reference.
Abbreviations: BNAT: Baseline assessment before neoadjuvant chemotherapy, consistent with the aforementioned studies.
chemotherapy; CI: Confidence interval; cN: Clinical lymph node staging ®
th
(American Joint Committee on Cancer [AJCC] 8 edition); cT: Clinical This suggests that the efficacy of Zercepac in treating
th
tumor size staging (AJCC 8 edition); cTNM: Tumor-node-metastasis HER2-positive breast cancer is essentially equivalent to
classification stage; HR: Hormone receptor; Ki-67: Antigen Kiel 67; that of Herceptin. Previous studies have also confirmed
5,13
OR: Odds ratio; Ref: Reference. ®
that neoadjuvant treatment combining Zercepac or
Herceptin with pertuzumab and chemotherapy yields
scale KRISTINE study compared six cycles of TCbHP comparable efficacy and safety profiles. Moreover, a model-
12
(docetaxel, carboplatin, trastuzumab, and pertuzumab) based economic evaluation in China in 2022 showed that
with ado-trastuzumab emtansine plus neoadjuvant Zercepac is more cost-effective than Herceptin, making
®
treatment. The tpCR rate in the TCbHP group was 55.7% it more accessible to patients and aligning with financial
(123/221), superior to the 44.4% (99/223) observed in the constraints in healthcare. 14
control group, thus validating both the efficacy and safety
®
of the TCbHP regimen. In our study, the overall tpCR rate In addition to these advantages, Zercepac offers other
among all patients was 50% (31/62). The highest tpCR rate benefits. Notably, it does not contain benzyl alcohol as a
was 55.2% (16/29), observed in the group treated with preservative, while the 440 mg formulation of Herceptin
®
Zercepac (a trastuzumab biosimilar) plus pertuzumab in available in China still does. Studies have shown that benzyl
combination with six cycles of docetaxel carboplatin (TCb) alcohol may reduce the efficacy of protein-based drugs and
Volume 9 Issue 3 (2025) 115 doi: 10.36922/EJMO025100044