Page 124 - EJMO-9-3
P. 124
Eurasian Journal of
Medicine and Oncology Zercepac + pyrotinib versus pertuzumab in HER2+ BC
®
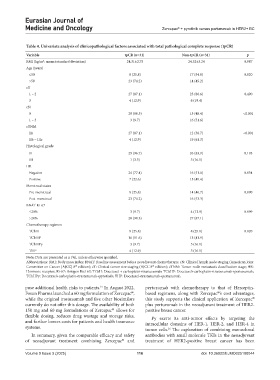
Table 4. Univariate analysis of clinicopathological factors associated with total pathological complete response (tpCR)
Variable tpCR (n=31) Non‑tpCR (n=31) p
BMI (kg/m ; mean±standard deviation) 24.31±2.75 24.32±3.24 0.987
2
Age (years)
≤50 8 (25.8) 17 (54.8) 0.020
>50 23 (74.2) 14 (45.2)
cT
1 – 2 27 (87.1) 25 (80.6) 0.490
3 4 (12.9) 6 (19.4)
cN
0 28 (90.3) 15 (48.4) <0.001
1 – 3 3 (9.7) 16 (51.6)
cTNM
IIa 27 (87.1) 12 (38.7) <0.001
IIb – IIIc 4 (12.9) 19 (61.3)
Histological grade
II 29 (96.7) 26 (83.9) 0.195
III 1 (3.3) 5 (16.1)
HR
Negative 24 (77.4) 16 (51.6) 0.034
Positive 7 (22.6) 15 (48.4)
Menstrual status
Pre-menstrual 8 (25.8) 14 (46.7) 0.090
Post-menstrual 23 (74.2) 16 (53.3)
BNAT Ki-67
≤20% 3 (9.7) 4 (12.9) 0.999
>20% 28 (90.3) 27 (87.1)
Chemotherapy regimen
TCbH 8 (25.8) 8 (25.8) 0.820
TCbHP 16 (51.6) 13 (41.9)
TCbHPy 3 (9.7) 5 (16.1)
THP 4 (12.9) 5 (16.1)
Note: Data are presented as n (%), unless otherwise specified.
Abbreviations: BMI: Body mass index; BNAT: Baseline assessment before neoadjuvant chemotherapy; cN: Clinical lymph node staging (American Joint
Committee on Cancer [AJCC] 8 edition); cT: Clinical tumor size staging (AJCC 8 edition); cTNM: Tumor-node-metastasis classification stage; HR:
th
th
Hormone receptor; Ki-67: Antigen Kiel 67; TCbH: Docetaxel + carboplatin+trastuzumab; TCbHP: Docetaxel+carboplatin+trastuzumab+pertuzumab;
TCbHPy: Docetaxel+carboplatin+trastuzumab+pyrotinib; THP: Docetaxel+trastuzumab+pertuzumab.
pose additional health risks to patients. In August 2022, pertuzumab with chemotherapy to that of Herceptin-
15
®
Fosun Pharma launched a 60 mg formulation of Zercepac , based regimens, along with Zercepac ’s cost advantages,
®
while the original trastuzumab and five other biosimilars this study supports the clinical application of Zercepac ®
currently do not offer this dosage. The availability of both plus pertuzumab in the neoadjuvant treatment of HER2-
150 mg and 60 mg formulations of Zercepac allows for positive breast cancer.
®
flexible dosing, reduces drug wastage and storage risks, Py exerts its anti-tumor effects by targeting the
and further lowers costs for patients and health insurance intracellular domains of HER-1, HER-2, and HER-4 in
systems. tumor cells. The exploration of combining monoclonal
16
In summary, given the comparable efficacy and safety antibodies with small molecule TKIs in the neoadjuvant
of neoadjuvant treatment combining Zercepac and treatment of HER2-positive breast cancer has been
®
Volume 9 Issue 3 (2025) 116 doi: 10.36922/EJMO025100044