Page 15 - ESAM-1-4
P. 15
Engineering Science in
Additive Manufacturing Experimental statistics in AM
A B
C
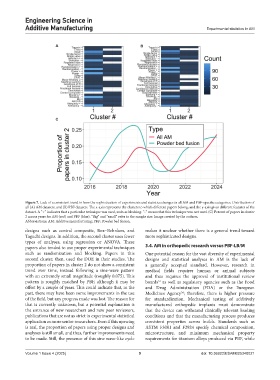
Figure 7. Lack of a consistent trend in how the sophistication of experiments and statistics changes in all AM and PBF-specific categories. Distribution of
all (A) AM datasets, and (B) PBF datasets. The x-axis represents the clusters to which different papers belong, and the y-axis gives different features of the
dataset. A “+” indicates that a particular technique was used, such as blocking. “-“ means that this technique was not used. (C) Percent of papers in cluster
2 across years for AM (red) and PBF (blue). “Big” and “small” refer to the sample size. Image created by the authors.
Abbreviations: AM: Additive manufacturing; PBF: Powder bed fusion.
designs such as central composite, Box–Behnken, and makes it unclear whether there is a general trend toward
Taguchi designs. In addition, the second cluster uses fewer more sophisticated designs.
types of analyses, using regression or ANOVA. These
papers also tended to use proper experimental techniques 3.4. AM in orthopedic research versus PBF-LB/M
such as randomization and blocking. Papers in this One potential reason for the vast diversity of experimental
second cluster, then, used the DOE in their studies. The designs and statistical analyses in AM is the lack of
proportion of papers in cluster 2 do not show a consistent a generally accepted standard. However, research in
trend over time, instead following a sine-wave pattern medical fields requires human or animal subjects
with an extremely small magnitude (roughly 0.075). This and thus requires the approval of institutional review
pattern is roughly matched by PBF, although it may be boards as well as regulatory agencies such as the Food
33
offset by a couple of years. This could indicate that, in the and Drug Administration (FDA) or the European
past, there may have been some improvements in the use Medicines Agency ; therefore, there is higher pressure
34
of the field, but any progress made was lost. The reason for for standardization. Mechanical testing of additively
that is currently unknown, but a potential explanation is manufactured orthopedic implants must demonstrate
the entrance of new researchers and new peer reviewers, that the device can withstand clinically relevant loading
publications that are not as strict in experimental statistical conditions and that the manufacturing process produces
application as more senior researchers. Even if this upswing consistent properties across builds. Standards such as
is real, the proportion of papers using proper designs and ASTM F3001 and F2924 specify chemical composition,
analyses is still small, and thus, further improvements need microstructure, and minimum mechanical property
to be made. Still, the presence of this sine wave-like cycle requirements for titanium alloys produced via PBF, while
Volume 1 Issue 4 (2025) 9 doi: 10.36922/ESAM025340021