Page 136 - GPD-4-2
P. 136
Gene & Protein in Disease Binding of 11q to DENV and WNV proteases
potent inhibitor of the DENV–NS2B–NS3 protease, with NS2B–NS3 protease active site. This suggests a stable and
implications for antiviral drug development. Per-residue persistent binding orientation of the ligand during the
decomposition of free energy suggests that the P132, dynamics process. The molecular interactions stabilizing
followed by S135 and Y161, significantly contributed to the 11q within the WNV protease included weak hydrogen
stability of 11q (Figure 5B). bonding interactions with T132 (36% occupancy) and
S135 (16% occupancy), which, although not dominant,
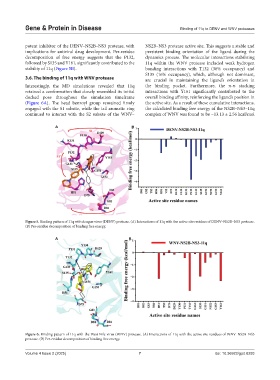
3.6. The binding of 11q with WNV protease are crucial in maintaining the ligand’s orientation in
Interestingly, the MD simulations revealed that 11q the binding pocket. Furthermore, the π-π stacking
retained a conformation that closely resembled its initial interactions with Y161 significantly contributed to the
docked pose throughout the simulation timeframe overall binding affinity, reinforcing the ligand’s position in
(Figure 6A). The head benzoyl group remained firmly the active site. As a result of these cumulative interactions,
engaged with the S1 subsite, while the tail aromatic ring the calculated binding free energy of the NS2B–NS3–11q
continued to interact with the S2 subsite of the WNV– complex of WNV was found to be −13.13 ± 2.56 kcal/mol
A B
Figure 5. Binding pattern of 11q with dengue virus (DENV) protease. (A) Interactions of 11q with the active site residues of DENV–NS2B–NS3 protease.
(B) Per-residue decomposition of binding free energy.
A B
Figure 6. Binding pattern of 11q with the West Nile virus (WNV) protease. (A) Interactions of 11q with the active site residues of WNV–NS2B–NS3
protease. (B) Per-residue decomposition of binding free energy.
Volume 4 Issue 2 (2025) 7 doi: 10.36922/gpd.8293