Page 38 - GTM-2-4
P. 38
Global Translational Medicine The research advances in HPV integration
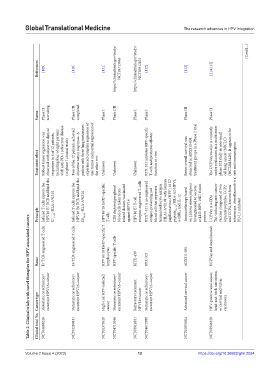
References [109] [110] [111] https://clinicaltrials.gov/study/ NCT04713046 https://clinicaltrials.gov/study/ NCT03912831 [112] [113] [114,115] (Cont’d...)
Status Phase II recruiting Phase I/II completed Phase I Phase I/II Phase I Phase I Phase III Phase II
Treatment effect Robust tumor regression was observed with objective clinical responses in 6 of 12 patients, including four of eight patients with anti-PD-1 refractory disease (in phase I clinical trials) Two of the 12 patients achieved objective tumor responses. A patient with three lung metastases experienced complete regression of one tumor and partial regression of the other two Unknown Unknown Unknown RTX-321 activates HPV-specific
Infused T cells express the HPV16 E7 TCR and bind the E7 11–19 -HLA-A*02:01 Infused T cells express the HPV16 E6 TCR and bind the E6 29–38 -HLA-A*02:01 HPV16/18 E6/E7-specific CD8 depleted peripheral blood cells taken from related donors vaccinated receptor-engineered T-cells RTX ™ -321 is an engineered antigen-presenting red blood cell that expresses human leukocyte antigen (HLA)-A*02:01 with human papillomavirus (HPV) 16 E7
Principle T cells against HPV16 HPV16 E7 T-cell 4-1BBL, and IL-12 protein UCPVax is a CD4 PD-L1 inhibitor
Table 2. Clinical trials with novel therapies for HPV‑associated cancers
E7 TCR-engineered T cells E6 TCR-engineered T cells HPV16/18 E6/E7-specific T HPV-specific T cells UCPVax and atezolizumab
Name lymphocytes KITE-439 RTX-321 ADXS11-001
Cancer type Metastatic or refractory/ recurrent HPV16+cancer Metastatic or refractory/ recurrent HPV16+cancer High-risk HPV-infected cancer Metastatic or refractory/ recurrent HPV16+cancer Refractory/recurrent HPV16+cancer Metastatic or refractory/ recurrent HPV16+cancer Advanced cervical cancer HPV-positive anal cancer, head-and-neck carcinoma, or cervical and vulvar carcinoma
Clinical trial No. NCT05686226 NCT02280811 NCT02379520 NCT04713046 NCT03912831 NCT04672980 NCT02853604 NCT03946358
Volume 2 Issue 4 (2023) 12 https://doi.org/10.36922/gtm.2034