Page 126 - GTM-4-1
P. 126
Global Translational Medicine AA amyloidosis in rheumatoid arthritis
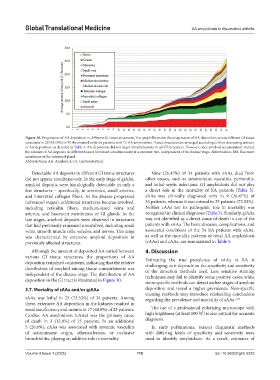
Figure 10. Progression of AA deposition in different GI tissue structures. The graph illustrates the progression of AA deposition across different GI tissue
structures in 29 (93.55%) of 31 rheumatoid arthritis patients with GI AA amyloidosis. Tissue structures are arranged according to their decreasing amount
of AA deposition, as detailed in Table 4. AA deposition did not begin simultaneously in all GI structures. However, once amyloid accumulation started,
the amount of AA deposits in different tissues increased simultaneously at a constant rate, independent of the disease stage. Abbreviation: BM: Basement
membrane of the intestinal gland.
Abbreviations: AA: Amyloid A; GI: Gastrointestinal.
Detectable AA deposits in different GI tissue structures Nine (26.47%) of 34 patients with sAAa died from
did not appear simultaneously. In the early stage of giAAa, other causes, such as autoimmune vasculitis, peritonitis,
amyloid deposits were histologically detectable in only a and lethal septic infections. GI amyloidosis did not play
few structures – specifically, in arterioles, small arteries, a direct role in the mortality of RA patients (Table 5).
and interstitial collagen fibers. As the disease progressed sAAa was clinically diagnosed only in 9 (26.47%) of
(advanced stages), additional structures became involved, 34 patients, whereas it was missed in 25 patients (73.53%).
including reticulin fibers, medium-sized veins and Neither cAAa nor its pathogenic role in mortality was
arteries, and basement membranes of GI glands. In the recognized in clinical diagnoses (Table 5). Similarly, giAAa
late stages, amyloid deposits were observed in structures was not identified as a direct cause of death in any of the
that had previously remained uninvolved, including small patients with sAAa. The basic diseases, complications, and
veins, smooth muscle cells, venules, and nerves. This stage associated conditions of the 34 RA patients with sAAa,
was characterized by extensive amyloid deposition in as well as the mortality patterns of renal AA amyloidosis
previously affected structures. (rAAa) and cAAa, are summarized in Table 5.
Although the amount of deposited AA varied between 4. Discussion
various GI tissue structures, the proportions of AA
deposition remained consistent, indicating that the relative Estimating the true prevalence of sAAa in RA is
distribution of amyloid among tissue compartments was challenging, as it depends on the specificity and sensitivity
of the detection methods used. Less sensitive staining
independent of the disease stage. The distribution of AA techniques may fail to identify some positive cases, while
deposition in the GI tract is illustrated in Figure 10. more specific methods can detect earlier stages of amyloid
3.7. Mortality of sAAa and/or giAAa deposition and reveal a higher prevalence. Non-specific
staining methods may introduce misleading conclusions
sAAa was lethal in 25 (73.53%) of 34 patients. Among regarding the prevalence and mortality of sAAa. 3,50
these, extensive AA deposition in the kidneys resulted in
renal insufficiency and uremia in 17 (68.0%) of 25 patients. The use of a professional polarizing microscope with
Cardiac AA amyloidosis (cAAa) was the primary cause high brightness (at least 100 W) is also critical for accurate
of death in 3 (12.0%) of 25 patients. In an additional diagnosis.
5 (20.0%), cAAa was associated with systemic vasculitis In early publications, various diagnostic methods
of autoimmune origin, atherosclerosis, or occlusive with differing levels of specificity and sensitivity were
bronchiolitis, playing an additive role in mortality. used to identify amyloidosis. As a result, estimates of
Volume 4 Issue 1 (2025) 118 doi: 10.36922/gtm.5325