Page 66 - IJB-1-1
P. 66
A novel 3D printing method for cell alignment and differentiation
distance between the grooves of 100 µm was applied.
As mentioned in the introduction, the purpose of
pre-etching grooves before cell seeding was to allow
the promotion of cell anisotropy and the regulation of
stem cell differentiation. In Figure 5, it can be clearly
seen that HDF cells (labeled with Calcein AM and
DAPI) have adopted a stretched morphology and are
aligning in the direction of the etched grooves on a
polystyrene culture plate (Figure 5(A)). In comparison,
the HDF cells on unmarked polystyrene appear more
dendritic and rhomboid (Figure 5(B)), hence the
grooves do have cell orientating effects.
Figure 5. Human dermal fibroblasts were elongated and
aligned on the etched grooves following direct seeding in tissue
culture medium onto the (A) etched polystyrene (B) compared
to seeding on unpatterned polystyrene. The fibroblasts were
stained with fluorescein diacetate (FDA) and 4',6-Diamidino-
2-Phenylindole, Dihydrochloride (DAPI) for visualization.
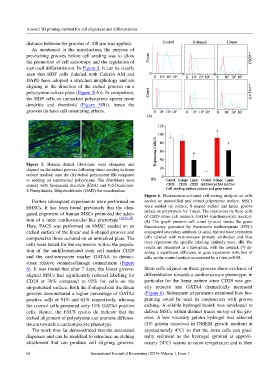
Figure 6. Fluorescence-activated cell sorting analysis on cells
Further subsequent experiments were performed on seeded on unmodified and etched polystyrene surface. MSCs
hMSCs. It has been found previously that the elon- were seeded on control, S-shaped etched and linear groove
gated alignment of human MSCs promoted the adop- etched on polystyrene for 7 days. The expression by these cells
of CD29 (stem cell marker), GATA4 (cardiomyocyte marker).
tion of a more cardiovascular-like phenotype [18,23,24] . (A) The graph presents cell count (y-axis) versus the green
Here, FACS was performed on hMSC seeded on an fluorescence generated by fluorescein isothiocyanate (FITC)
etched surface of the linear and S-shaped grooves and conjugated secondary antibody (x-axis), the red trace represents
compared to those cultured on an unmarked plate. The cells labeled with non-immune primary antibodies and blue
cells were tested for the expression within the popula- trace represents the specific labeling antibody trace. (B) The
results are presented as a histogram, with the asterick (*) de-
tion of the undifferentiated stem cell marker CD29 noting a significant difference in gene expression with that of
and the cardiomyocyte marker GATA4, to demon- cells on the control surface as assessed by a t-test p<0.05.
strate relative stemness/lineage commitment (Figure
6). It was found that after 7 days, the linear groove- Stem cells aligned on these grooves show evidence of
aligned MSCs had significantly reduced labelling for differentiation towards a cardiomyocyte phenotype, in
CD29 at 39% compared to 92% for cells on the particular for the linear pattern since CD29 was gre-
un-patterned surface. Both the S-shaped and the linear atly reduced and GATA4 dramatically increased
grooves demonstrated a higher percentage of GATA4 (Figure 6). Subsequent experiments examined how bio-
positive cells at 91% and 62% respectively, whereas printing could be used in conjunction with groove
the control cells presented only 11% GATA4 positive etching. A soluble hydrogel bioink was developed to
cells. Hence, the FACS results do indicate that the deliver MSCs within distinct traces on top of the gro-
etched alignment of polystyrene can promote differen- oves. A low viscosity gelatin hydrogel was selected
tiation towards a cardiomyocyte phenotype. (2% gelatin dissolved in DMEM growth medium at
The work thus far demonstrated that the automated approximately 4°C) so that the stem cells can grad-
dispenser unit can be modified to introduce an etching ually sediment as the hydrogel (printed at approxi-
attachment that can produce cell aligning grooves. mately 24°C) warms to room temperature and is then
62 International Journal of Bioprinting (2015)–Volume 1, Issue 1