Page 326 - IJB-10-1
P. 326
International Journal of Bioprinting 3D-bioprinted meningioma model
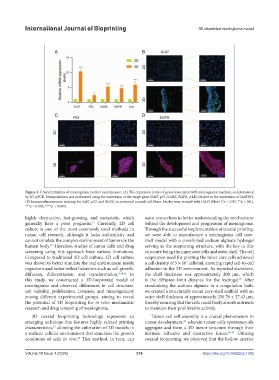
Figure 3. Characterization of meningioma marker maintenance. (A) The expression levels of genes associated with meningioma markers, as determined
by RT-qPCR. Normalization was performed using the expression of the target gene (Ki67, p53, KARS, EGFR, ALK) relative to the expression of GAPDH.
(B) Immunofluorescence staining for Ki67, p53, and EGFR in sectioned coaxial cell fibers. Nuclei were stained with DAPI (blue) (*p < 0.05, **p < 0.01,
***p < 0.005, ****p < 0.001).
highly destructive, fast-growing, and metastatic, which assist researchers in better understanding the mechanisms
generally have a poor prognosis. Currently, 2D cell behind the development and progression of meningioma.
21
culture is one of the most commonly used methods in Through the successful implementation of coaxial printing,
tumor cell research, although it lacks authenticity and we were able to manufacture a meningioma cell core-
cannot simulate the complex environment of tumors in the shell model with a crosslinked sodium alginate hydrogel
human body. Therefore, studies of tumor cells and drug serving as the supporting structure, with the key to this
22
screening using this approach have various limitations. structure being the inner core cells and outer shell. The cell
Compared to traditional 2D cell culture, 3D cell culture suspension used for printing the inner core cells achieved
was shown to better simulate the real environment inside a cell density of 5 × 10 cells/ml, ensuring rapid cell-to-cell
7
organisms and better reflect behaviors such as cell growth, adhesion in the 3D environment. As reported elsewhere,
diffusion, differentiation, and transformation. 9,14,16 In the shell thickness was approximately 200 μm, which
this study, we constructed a 3D-bioprinted model of is the diffusion limit distance for the hydrogel. After
25
meningioma and observed differences in cell structure, crosslinking the sodium alginate in a coagulation bath,
cell viability, proliferation, invasion, and tumorigenicity we created a structurally sound core-shell scaffold with an
among different experimental groups, aiming to reveal outer shell thickness of approximately 250.79 ± 27.42 μm,
the potential of 3D bioprinting for in vitro mechanistic thereby ensuring that the cells could freely absorb nutrients
research and drug screening of meningioma. to maintain their proliferative activity.
3D coaxial bioprinting technology represents an Tumor cell self-assembly is a crucial phenomenon in
26
emerging technique that features highly refined printing tumor development, wherein tumor cells spontaneously
23
characteristics, allowing the cultivation of 3D models in aggregate and form a 3D tumor structure through their
a realistic cellular environment that simulates the growth intrinsic adhesive and interactive forces. 26-27 Utilizing
conditions of cells in vivo. This method, in turn, can coaxial bioprinting, we observed that the hollow interior
24
Volume 10 Issue 1 (2024) 318 https://doi.org/10.36922/ijb.1342