Page 493 - IJB-10-1
P. 493
International Journal of Bioprinting Endothelial monolayer formation on scaffolds
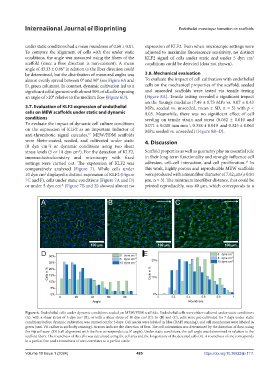
under static conditions had a mean roundness of 0.60 ± 0.01. expression of KLF2. Even when microscopic settings were
To compare the alignment of cells with that under static adjusted to maximize fluorescence sensitivity, no distinct
conditions, the angle was measured using the fibers of the KLF2 signal of cells under static and under 5 dyn cm 2
scaffold (since a flow direction is non-existent). A mean conditions could be detected (data not shown).
angle of 43.33 ± 0.86° in relation to the fiber direction could
be determined, but the distribution of measured angles was 3.8. Mechanical evaluation
almost evenly spread between 0° and 90° (see Figure 6A and To evaluate the impact of cell cultivation with endothelial
D, green columns). In contrast, dynamic cultivation led to a cells on the mechanical properties of the scaffold, seeded
significant cell alignment with almost 50% of all cells exposing and unseeded scaffolds were tested via tensile testing
an angle of >20° relative to the medium flow (Figure 6D). (Figure 8A). Tensile testing revealed a significant impact
on the Young’s modulus (7.49 ± 0.73 MPa vs. 9.07 ± 0.43
3.7. Evaluation of KLF2 expression of endothelial MPa, seeded vs. unseeded, mean ± SD, n = 5) with p <
cells on MEW scaffolds under static and dynamic 0.05. Meanwhile, there was no significant effect of cell
conditions seeding on tensile strain and stress (0.062 ± 0.010 and
To evaluate the impact of dynamic cell culture conditions 0.071 ± 0.008 mm mm ; 0.358 ± 0.019 and 0.325 ± 0.063
-1
on the expression of KLF2 as an important inductor of MPa; seeded vs. unseeded) (Figure 8B–D).
anti-thrombotic signal cascades, MEW/FDM scaffolds
22
were fibrin-coated, seeded, and cultivated under static 4. Discussion
(0 dyn cm ) or dynamic conditions using two shear
-2
stress levels (5 or 10 dyn cm ). For the detection of KLF2, Scaffold properties as well as geometry play an essential role
2
immunohistochemistry and microscopy with fixed in their long-term functionality and strongly influence cell
18
settings were carried out. The expression of KLF2 was adhesion, cell–cell interaction, and cell proliferation. In
comparatively analyzed (Figure 7). While cells under this work, highly porous and reproducible MEW scaffolds
10 dyn cm displayed a distinct expression of KLF2 (Figure were produced with a mean fiber diameter of 7.62 µm (± 0.84
2
7C and F), cells under static conditions (Figure 7A and D) µm, n = 3). The minimum interfiber distance, that could be
or under 5 dyn cm (Figure 7B and E) showed almost no printed reproducibly, was 40 µm, which corresponds to a
-2
Figure 6. Endothelial cells under dynamic conditions seeded on MEW/FDM scaffolds. Endothelial cells were either cultured under static conditions
(A), with a shear stress of 5 dyn cm (B), or with a shear stress of 10 dyn cm (C). In (B) and (C), cells were pre-cultivated for 7 days under static
2
2
conditions before dynamic cultivation was carried out for 3 days. Cell nuclei were labeled in blue (DAPI staining), and cell membranes were labeled in
green (anti-VE cadherin antibody staining). Arrows indicate the direction of flow. The cell orientation was determined by the direction of flow, using
the Fiji software (D) (cell alignment with the flow corresponds to a 0° angle). Under static conditions, the cell angle was determined in relation to the
scaffold fibers. The roundness of the cells was calculated using the cell area and the longest axis of the detected cells (E). A roundness of one corresponds
to a perfect line and a roundness of zero correlates to a perfect circle.
Volume 10 Issue 1 (2024) 485 https://doi.org/10.36922/ijb.1111