Page 65 - IJB-10-1
P. 65
International Journal of Bioprinting Microfluidic-assisted 3D bioprinting
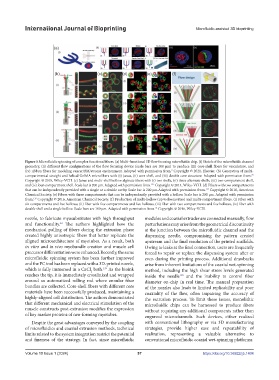
Figure 5.Microfluidic spinning of complex functional fibers. (a) Multi-functional 3D flow focusing microfluidic chip. (i) Sketch of the microfluidic channel
geometry, (ii) different flow configurations of the flow focusing device (scale bars are 100 μm) to produce (iii) core-shell fibers for vasculature, and
(iv) ribbon fibers for modeling cancer/BM/stroma environment. Adapted with permission from. Copyright © 2021, Elsevier. (b) Generation of multi-
3
compartmental straight and helical GelMA microfibers with (i) Janus, (ii) core-shell, and (iii) double core structure. Adapted with permission from.
73
Copyright © 2018, Wiley-VCH. (c) Janus and multi-shell hollow alginate fibers with (i) two shells, (ii) three alternate shells, (iii) two-compartment shell,
and (iv) four-compartment shell. Scale bar is 200 μm. Adapted with permission from. Copyright © 2014, Wiley-VCH. (d) Fibers with two compartments
135
that can be independently provided with a single or a double cavity. Scale bar is 200 μm.Adapted with permission from. Copyright © 2016, American
115
Chemical Society. (e) Fibers with three compartments that can be independently provided with a hollow. Scale bar is 200 μm. Adapted with permission
from. Copyright © 2016, American Chemical Society. (f) Production of multi-hollow (up to fivecavities) and multi-compartment fibers. (i) Fiber with
115
six compartments and five hollows, (ii) fiber with five compartments and five hollows, (iii) fiber with two compartments and five hollows, (iv) fiber with
double shell and a single hollow. Scale bars are 100 µm. Adapted with permission from. Copyright © 2016, Wiley-VCH.
74
nozzle, to fabricate myosubstitutes with high throughput modules and coaxial extruder are connected manually, flow
and functionality. The authors highlighted how the perturbations may arise from the geometrical discontinuity
63
mechanical pulling of fibers during the extrusion phase at the junction between the microfluidic channel and the
created highly anisotropic fibers that better replicate the dispensing needle, compromising the pattern created
aligned microarchitecture of myotubes. As a result, both upstream and the final resolution of the printed scaffolds.
in vitro and in vivo myobundle creation and muscle cell Owing to leaks at the final connection, users are frequently
precursor differentiation were enhanced. Recently, the same forced to repair or replace the dispensing system after or
microfluidic spinning system has been further improved even during the printing process. Additional drawbacks
and the PC tool has been replaced with a 3D-printed nozzle, arise from inherent limitations of the coaxial wet-spinning
which is fully immersed in a CaCl bath. As the bioink method, including the high shear stress levels generated
139
2
reaches the tip, it is immediately crosslinked and wrapped inside the needle and the inability to control fiber
140
around an automatized rolling rod where annular fiber diameter on-chip in real time. The manual preparation
bundles are collected. Core-shell fibers with different core of the nozzles also leads to limited replicability and poor
materials have been successfully produced, maintaining a coaxiality of the flow, often impairing the accuracy of
highly-aligned cell distribution. The authors demonstrated the extrusion process. To limit these issues, monolithic
that different mechanical and electrical stimulation of the microfluidic chips can be harnessed to produce fibers
muscle constructs post-extrusion modifies the expression without requiring any additional components rather than
of key marker proteins of neo-forming myotubes. engraved microchannels. Such devices, either realized
Despite the great advantages conveyed by the coupling with conventional lithography or via 3D manufacturing
of microfluidics and coaxial extrusion methods, technical strategies, provide higher ease and repeatability of
limits related to the system integration restrict the potential realization, representing a valuable alternative to
and fineness of the strategy. In fact, since microfluidic conventional microfluidic coaxial wet-spinning platforms.
Volume 10 Issue 1 (2024) 57 https://doi.org/10.36922/ijb.1404