Page 69 - IJB-10-1
P. 69
International Journal of Bioprinting Microfluidic-assisted 3D bioprinting
of the fibers, while endothelial cells were perfused within 4.3. 3D microfluidic bioprinting: enhancing the
the lumen. Eventually, the formation of a functional and complexity of 3D constructs
sealed conduit was confirmed by the expression of specific The use of low-viscosity inks has conveyed manifold
target genes and perfusion tests (Figure 7b). By creating a advantages, among which the possibility to process
triple coaxial flow of different materials, it is also possible hydrogel precursors within microfluidic channels before
to generate more complex fibers, such as tri-layered core- extrusion (Figure 6c). Coupling microfluidic operators
shell fibers 174,175 or branched microfibers. 176 upstream of the extrusion printhead enables the 3D
manufacturing of complex scaffolds tailored to their micro-
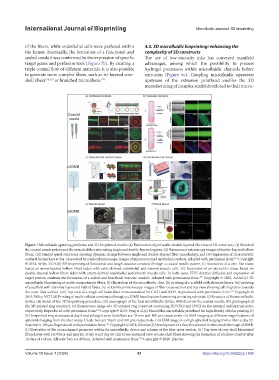
Figure 7.Microfluidic spinning platforms and 3D-bioprinted models.(a) Fabrication of perfusable double-layered fiber-based 3D constructs. (i) Sketch of
the coaxial nozzle system and the extruded fiber alternating single and double-layered regions, (ii) fluorescence microscopy images of double-layered hollow
fibers, (iii) printed spiral structures showing dynamic change between single and double-layered fiber morphology, and (iv) expression of characteristic
urethral biomarkers at day 14 revealed by confocal microscopy images of immunostained urothelial conduits. Adapted with permission from. Copyright
121
© 2018, Wiley-VCH.(b) 3D bioprinting of functional and tough vascular conduits through a coaxial needle system. (i) Recreation of a vein-like tissue
based on mono-layered hollow fibers laden with veins-derived endothelial and smooth muscle cells. (ii) Recreation of an artery-like tissue based on
double-layered hollow fibers laden with artery-derived endothelial and smooth muscle cells. In both cases, FITC-dextran diffusion and expression of
target protein confirms the formation of a sealed and functional vascular conduit. Adapted with permission from. Copyright © 2022, AAAS.(c) 3D
173
microfluidic bioprinting of multi-compartment fibers. (i) Illustration of the microfluidic chip, (ii) printing of a scaffold with alternate layers, (iii) printing
of a scaffold with alternate layers and hybrid fibers, (iv, v) Confocal microscopy images of fiber cross-section and top view showing cell migration towards
the outer fiber surface. (vii) Top view of a single cell laden fiber immunostained for CD31 and DAPI. Reproduced with permission from. Copyright ©
123
2015, Wiley-VCH.(d) Printing of multi-cellular constructs through a c3DMB based system harnessing a rotating substrate. (i) Structure of the microfluidic
device, (ii) sketch of the 3D bioprinting procedure, (iii) macrograph of the final microfluidic device, with focus on the coaxial needle, (iv) photograph of
the 3D-printed ring construct, (v) fluorescence image of a 3D-printed ring construct containing HUVECs and H9C2 on the external and internal parts,
respectively. Reproduced with permission from. Copyright © 2019, Feng et al.(e) Monolithic microfluidic printhead for high density cellular printing.(i)
124
3D-bioprinted ring structures at day 0 and enlarged view. Scale bars are 10 mm and 100 μm, respectively. (ii) SEM imaging at different magnifications of
spheroids bulging from the fiber at day 3.Scale bars are 50 μm and 100 μm, respectively. (iii) SEM image of a single spheroid bulging from a fiber at day 12.
Scale bar is 100 μm.Reproduced with permission from. Copyright © 2018, Elsevier.(f) Development of a functional renal in vitro model through a3DMB.
191
(i) Illustration of the microchannel geometry within the microfluidic device and scheme of the fiber cross-section. (ii) Top view of core-shell bioprinted
fibers laden with HUVECs and pmTEC at day 0 and day 14. (iii) Cross-sectional view of core-shell fibers showing the formation of a hollow conduit after
14 days of culture. All scale bars are 200 μm. Adapted with permission from. Copyright © 2020, Elsevier.
196
Volume 10 Issue 1 (2024) 61 https://doi.org/10.36922/ijb.1404